Increased risk of recurrence associated with certain risk factors in breast cancer patients after DIEP-flap reconstruction and lipofilling—a matched cohort study with 200 patients
Introduction
Lipofilling for contour corrections or volume restorations after breast reconstruction has emerged to be a standard in common practice (1). Sixty-two percent of surveyed members of the American Society of Plastic Surgeons (ASPS) use this technique for breast reconstruction purposes for which volume deficiencies especially in the upper medial quadrant of the reconstructed breast are the most common indication (2). Despite of fat being biocompatible, non-immunogenic and a natural filler, concerns on its oncological safety persist in breast cancer patients undergoing mastectomy and reconstruction (3-6). In vitro studies have shown the lipoaspirate to be a bioactive substance whose adipose-derived stem cells (ADSCs) are capable of cell stimulation and tissue regeneration by the secretion of numerous factors (7-9) that also promote cancer growth, angiogenesis and alter the antitumor immune response (10) when in close vicinity to breast cancer cells. Thus experimental studies demonstrated a potential risk of lipofilling to cause cancer recurrence as ADSCs may stimulate quiescent cancer cells still resident after surgery (11-13). Particularly one retrospective study points to an increased risk for locoregional recurrence after mastectomy for ductal carcinoma in situ (DCIS) or breast conservative therapy (5,6). In 2007 the ASPS declared a Fat Graft Task Force that strongly emphasized the need for more research to prove oncological safety of lipofilling (14), in 2012 the Patient Safety Committee demanded for more evidence-based guidance for the safety of fat grafting to the post-mastectomy reconstructed breast (15). Up to date, there is a limited amount of studies showing evidence of lipofilling to be oncological safe. Recently a multicenter case-cohort study (16) and a matched controlled study (17) demonstrated that fat transfer in breast cancer patients who underwent mastectomy, was not associated with a higher risk of cancer recurrence. Yet, none of the studies so far, has focused on determining and analyzing potential patient risk factors that could work in synergy with the lipoaspirate and might increase the risk of cancer recurrence. Up to date, risk factors for recurrence in breast cancer patients undergoing a delayed deep inferior epigastric perforator (DIEP)-flap reconstruction and subsequent lipofilling have not been assessed in the literature. Further, very little is known about the interaction of ADSCs and dormant cancer cells in form of occult (micro) metastases. Thus, the purpose of this study is to identify potential risk factors for this patient cohort and improve patient selection for fat grafting procedures.
Methods
Study population
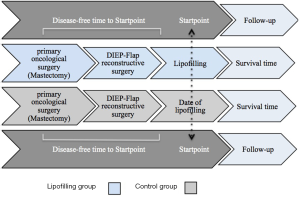
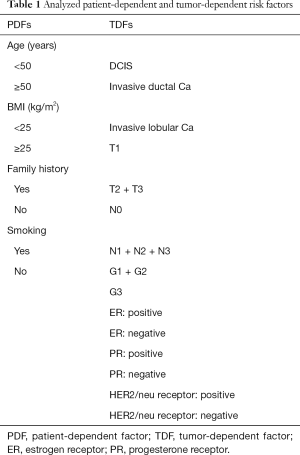
The study is a matched retrospective cohort study. All patients that underwent a lipofilling between 2009 and 2013 at the Department of Plastic and Reconstructive Surgery at the SANA Klinik in Düsseldorf were identified through a prospectively maintained hospital database. Only breast cancer patients treated with total mastectomy and delayed DIEP-flap reconstruction were eligible. Patients with immediate breast reconstruction, bilateral breast cancer, other reconstructive method than the DIEP-flap, or prophylactic mastectomy were excluded. Further, patients were not allowed to have a cancer recurrence between the time interval of their primary surgery (mastectomy), their delayed DIEP-flap reconstruction and their lipofilling (Figure 1). In total, one hundred patients met the inclusion criteria and were selected for the study. For each of these patients, a control patient was selected from the same database. A matched control patient underwent a total mastectomy for breast cancer and a delayed breast reconstruction with the DIEP-flap, but did not undergo subsequent lipofilling and was recurrence-free from the primary oncological surgery up to the startpoint of the study follow-up (Figure 1). The patient match was performed 1:1 and included following categorical characteristics: age (within 5 years), year of primary oncological surgery (within 3 years), year of DIEP-flap reconstructive surgery (within 3 years), primary tumor histopathology (DCIS, invasive lobular, invasive ductal), receptor status (estrogen, progesterone, Her-2/neu), tumor stage (TNM) and grade (G). Further, risk factors for cancer recurrence were determined from the literature and according to these, the whole study population was subdivided into risk factor-subgroups, which were categorized as patient-dependent-factors (PDFs) and tumor-dependent-factors (TDFs) (Table 1). The study-specific follow-up started for each matched lipofilling and control patient with the date of the lipofilling procedure. This was labeled as the “startpoint” (Figure 1). A recurrence was defined as an event involving local, regional or distant relapse. The detection of a recurrence or death was considered the end of the follow-up period. To control and confirm breast cancer recurrence, a questionnaire to each patient was sent out, addressing questions if, when and where a recurrence has occurred. An informed consent was also included in the letter. When the patient did not respond, she was contacted by telephone. In case of unavailability, she was considered lost-to-follow-up and marked as “not available”.
Full table
Fat grafting technique
In all patients the fat harvesting technique after Coleman was used (18-21).
Statistical analyses
All statistical analyses were performed using the R software (The R development Core Team 2004; Free Software Foundation, Boston, MA) and the SAS package (SAS Institute, Cary, NC). Chi-square test was performed to assess potential differences between the lipofilling and control groups and to ensure homogeneity. The Log-Rank test was used to compare the follow-up times in different subgroups and the results are presented in Kaplan-Meier curves. Univariate Cox proportional hazard regression models were used to evaluate the association of potential risk factors (PDFs, TDFs) with the time to recurrence. The results were expressed as hazard ratios (HR) with 95% confidence interval (CI). All tests were two-sided and P-values below 0.05 were considered as statistically significant.
Results
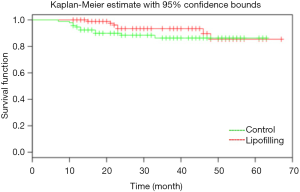
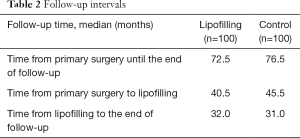
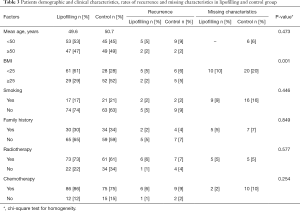
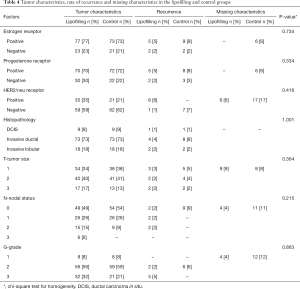
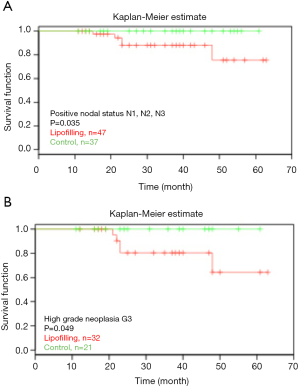
The median follow-up from the date of mastectomy until the end of follow up in the lipofilling and control groups was 72.5 months (range 25–173 months) and 76.5 months (range 28–151 months), respectively. The median follow-up from the startpoint was 32 months (range 11–67 months) in the lipofilling and 31 months (range 7–63 months) in the control group (Table 2). The median age in both groups was around 50 years. There were 29% overweight patients in the lipofilling group and 52% in the control group. More patients in our population were non-smoking and had a negative family history of breast cancer (17% and 21% smokers, 30% and 34% with positive family history in the lipofilling and the control group, respectively). Around 80% received chemotherapy and more than half received radiotherapy (Table 3). A total of 182 of the tumors were invasive and only 18 were DCIS (Table 4). Node-positive disease was in 47% and 35%, tumor size T2-T3 in 57% and 54%, grade G2-G3 in 88% and 80% patients in the lipofilling and the control group, respectively. Tumor morphology was equal in the both group: 9% DCIS, 73% invasive ductal and 18% invasive lobular cancer. Receptor status in the lipofilling group vs. the control group was as follows: ER-positive 77% vs. 73%, PR-positive 70% vs. 72%, Her2/neu-positive 35% vs. 21%. The overall recurrence rate is 13% in the lipofilling group and 12% in the control. Seven and eleven patients had a recurrence event in the lipofilling and control group, respectively, presenting with comparable survival rates (HR =0.57, 95% CI: 0.22–1.47, P=0.24) (Figure 2). Among the 7 lipofilling patients with recurrence, there were 4 invasive ductal carcinomas, 2 invasive lobular carcinomas and 1 DCIS. In the control group, recurrence included 8 invasive ductal carcinomas, 2 invasive lobular carcinomas and 1 DCIS (Table 4). The subgroup survival analysis by the means of the log rank test showed that lipofilling increased the risk of recurrence in women that had a breast cancer with a high-grade neoplasia (G3) (P=0.049) and a positive nodal status (P=0.035) (Figure 3).
Full table
Full table
Full table
Discussion
In contrast to previous studies that have analyzed the cancer recurrence risk of fat grafting in patients that underwent mastectomy/partial mastectomy with either prosthetic or autologous breast reconstruction, this study focuses only on breast cancer patients after mastectomy with a delayed DIEP-flap reconstruction. Up to date, this study is the only one addressing risk factors for this particular study cohort. The overall analysis of the oncological safety of lipofilling in breast cancer patients after delayed DIEP-flap reconstruction following mastectomy does not show any significant difference in the DFS between the lipofilling and control group. This finding is in concordance with the estimated HR stating that lipofilling poses no risk of recurrence on the mentioned study population. According to past literature, a few studies have evaluated the oncological safety and follow-up in patients with breast cancer who underwent lipofilling following mastectomy and reconstruction resulting in not congruent results. Thus, the evidence of oncological safety still remains uncertain, especially when 49% of surveyed members of the ASPS stated that the lack of evidence concerning the impact lipofilling has on breast cancer development or recurrence withholds them to practice the method (2). Petit et al. (5) was the first to discuss the controversy about the safety of lipofilling in breast cancer patients. Like in this present study, they matched patients who underwent lipofilling with control patients that did not obtain lipofilling. However, while Petit et al. performed the study on a population having both breast conserving surgery (39%) and mastectomy (61%), the patient population in this study addressed only patients after mastectomy undergoing a delayed breast reconstruction with a DIEP-flap, making it more homogenous. Nevertheless, their findings were similar to the ones in this study with regards to the overall survival and recurrence rate. Petit et al. presented an overall recurrence rate of 4% in the lipofilling group and 3% in the control group. A similar recurrence incidence can be also found in the present study where the overall recurrence rate is 13% in the lipofilling group and 12% in the control. Yet, noticeable is the difference of 9% between the results of the two studies. This could be explained by the fact that it is inconclusive in Petit’s analysis what type of reconstruction (prosthesis or autologous tissue) patients after mastectomy underwent prior to lipofilling. In this study population all patients underwent an autologous tissue reconstruction after their mastectomy. Taking this background into account, the recurrence rate can be already influenced by this fact in comparison with patients having only mastectomy (22) or an implant-based reconstruction. Recently two papers (16,17) have emerged assessing the overall risk of fat grafting on a large scale study population and they also support the oncological safety of the procedure in breast reconstruction. Yet, in one of them (16) patients below 50 years, with a Ki-67 value greater than or equal to 14 and a high-grade neoplasia were associated with an increased recurrence rate after lipofilling. Also in the study by Petit et al. (6) concerning fat grafting safety in patients with only intra-epithelial neoplasia, patients below 50 years, a Ki-67 value greater than or equal to 14 and a high-grade neoplasia were at increased risk of local recurrence following fat grafting. Also the subgroup analysis in this study shows patients with a high-grad neoplasia (G3) or a positive nodal status to be more susceptible to an increased risk of recurrence after breast reconstruction induced by lipofilling.
Grading
The recurrence rate for G3 neoplasias in the study by Petit and his colleagues resulted in 19% for the lipofilling group (3 events) and no events in the control group. This result is in line with the findings in our study, since lipofilling patients with a high-grade neoplasia had a recurrence rate of 16% (5 events) in comparison with the control group that presented with no events. Generally, poorly differentiated cancers are proven to have a worse patient survival than low-grade malignancies. This can be seen in studies such as the one by Bijker et al. (23) where patients with DCIS were treated by local excision with or without consecutive radiation. Factors significantly associated with an increased local recurrence risk were poorly differentiated DCIS. In the randomized analysis by Johansen et al. (24), patients with Grade 2–3 breast cancer had a worse survival than patients with Grade 1 tumors. Thus, the higher the Grade, the more aggressive are the tumor cells (25) and therefore in theory eventually easier susceptible to environmental cues such as lipofilling, if any would remain after surgery in form of occult micrometastases or as dormant cell in metastatic niches (26).
Nodal status
The nodal status is one of the most important prognostic factors predictive of recurrence and survival in breast cancer (27). There is a direct positive relationship between the number of involved axillary lymph nodes and the risk for distant recurrences (28). As the number of lymph nodes metastases increases, the risk of cancer recurrence increases and the survival decreases (29). For breast cancer cells to metastasize, they must take either a haematogenous or lymphatic route to disseminate to the tissues. In case of haematogenous spread, the cells have to pass through the basement membrane to relocate themselves. During this movement, they can get trapped in the endothelium of the microvasculature which constitutes a niche that induces cancer cell quiescence/dormancy via thrombospondin-1 (TSP-1). Yet, once growth of the vasculature is induced, the endothelium looses its tumor-suppressive nature by the reduced expression of TSP-1 and enhanced expression of periostin (POSTN) and transforming growth factor-β1 (TGF-β1) that promote neovascularization but at the same time activate the cancer cells to proliferate (26,30,31). ADSCs secrete a number of vascular endothelial growth factors (VEGFs) that induce neovascularization but can also stimulate lymphangiogenesis (32). Thus, fat grafting might be indirectly able to reactive dormant cancer cells in their niche by causing the vasculature to grow. Patients with a known node positive disease have an increased risk of occult micro-metastases hidden in their lymph nodes (33,34). If similar mechanism applies to dormant breast cancer cells in lymphnodes/lymphatic vessels as in the vasculature, then lipofilling that is applied in the near vicinity of such lymph nodes, might in theory, initiate proliferation of the dormant cancer cells and therefore provoke a recurrence through VEGFs released by the adipocytes and ADSCs. There are only five recurrences in the whole node positive study population (lipofilling and control) and they are found to be only in the lipofilling group, potentially being stimulated by the lipoaspirate.
The strengths of the study lie in its matched cohort design and homogeneous patient population. The retrospective nature of the study and insufficient information about adjuvant therapy and mastectomy technical aspects are a limitation. It should be taken into consideration that the results from the subgroup analysis are exploratory and require further validation.
Conclusions
In summary, lipofilling does not seem to increase the risk of breast cancer recurrence more than a DIEP-tissue flap. Thus, autologous fat grafting remains an attractive proposition for women wishing to optimize their aesthetic outcomes after breast reconstruction with a free flap. However, the subgroup analysis revealed a node positive disease and/or a high grade neoplasia to increase the risk of recurrence when they coexisted with the lipoaspirate. Yet, before withholding fat grafting as a reconstruction option in the above mentioned patient groups, more randomized controlled studies with a larger sample size would be required to determine clinical significance and re-evaluate the need to improve patient selection for fat grafting procedures. Further, tumor dormancy awakening remains very poorly understood and requires additional studies, especially in regards to fat grafting. Currently, there are sparse publications addressing this topic.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The work has been approved by the ethical committee of the University of Freiburg, and the ID number of the ethics approval is 526/15.
References
- Spear SL. Fat for breast: where are we? Plast Reconstr Surg 2008;122:983-4. [Crossref] [PubMed]
- Kling RE, Mehrara BJ, Pusic AL, et al. Trends in autologous fat grafting to the breast: a national survey of the american society of plastic surgeons. Plast Reconstr Surg 2013;132:35-46. [Crossref] [PubMed]
- Bertolini F, Lohsiriwat V, Petit JY, et al. Adipose tissue cells, lipotransfer and cancer: a challenge for scientists, oncologists and surgeons. Biochim Biophys Acta 2012;1826:209-14. [PubMed]
- Bertolini F, Petit JY, Kolonin MG. Stem cells from adipose tissue and breast cancer: hype, risks and hope. Br J Cancer 2015;112:419-23. [Crossref] [PubMed]
- Petit JY, Botteri E, Lohsiriwat V, et al. Locoregional recurrence risk after lipofilling in breast cancer patients. Ann Oncol 2012;23:582-8. [Crossref] [PubMed]
- Petit JY, Rietjens M, Botteri E, et al. Evaluation of fat grafting safety in patients with intraepithelial neoplasia: a matched-cohort study. Ann Oncol 2013;24:1479-84. [Crossref] [PubMed]
- Akita S, Akino K, Hirano A, et al. Noncultured autologous adipose-derived stem cells therapy for chronic radiation injury. Stem Cells Int 2010;2010:532704. [Crossref] [PubMed]
- Akita S, Yoshimoto H, Akino K, et al. Early experiences with stem cells in treating chronic wounds. Clin Plast Surg 2012;39:281-92. [Crossref] [PubMed]
- Phulpin B, Gangloff P, Tran N, et al. Rehabilitation of irradiated head and neck tissues by autologous fat transplantation. Plast Reconstr Surg 2009;123:1187-97. [Crossref] [PubMed]
- Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007;449:557-63. [Crossref] [PubMed]
- Perrot P, Rousseau J, Bouffaut AL, et al. Safety concern between autologous fat graft, mesenchymal stem cell and osteosarcoma recurrence. PLoS One 2010;5:e10999. [Crossref] [PubMed]
- Pearl RA, Leedham SJ, Pacifico MD. The safety of autologous fat transfer in breast cancer: lessons from stem cell biology. J Plast Reconstr Aesthet Surg 2012;65:283-8. [Crossref] [PubMed]
- Martin-Padura I, Gregato G, Marighetti P, et al. The white adipose tissue used in lipotransfer procedures is a rich reservoir of CD34+ progenitors able to promote cancer progression. Cancer Res 2012;72:325-34. [Crossref] [PubMed]
- Gutowski KA. ASPS Fat Graft Task Force. Current applications and safety of autologous fat grafts: a report of the ASPS fat graft task force. Plast Reconstr Surg 2009;124:272-80. [Crossref] [PubMed]
- American Society of Plastic Surgeons. Post-mastectomy fatgraft/fat transfer ASPS guiding principles. Available online: https://www.plasticsurgery.org/Documents/Health-Policy/Principles/principle-2015-post-mastectomy-fat-grafting.pdf
- Myckatyn TM, Wagner IJ, Mehrara BJ, et al. Cancer Risk after Fat Transfer: A Multicenter Case-Cohort Study. Plast Reconstr Surg 2017;139:11-18. [Crossref] [PubMed]
- Kronowitz SJ, Mandujano CC, Liu J, et al. Lipofilling of the Breast Does Not Increase the Risk of Recurrence of Breast Cancer: A Matched Controlled Study. Plast Reconstr Surg 2016;137:385-93. [Crossref] [PubMed]
- Coleman SR. Facial augmentation with structural fat grafting. Clin Plast Surg 2006;33:567-77. [Crossref] [PubMed]
- Coleman WP 3rd, Glogau RG, Klein JA, et al. Guidelines of care for liposuction. J Am Acad Dermatol 2001;45:438-47. [Crossref] [PubMed]
- Kurita M, Matsumoto D, Shigeura T, et al. Influences of centrifugation on cells and tissues in liposuction aspirates: optimized centrifugation for lipotransfer and cell isolation. Plast Reconstr Surg 2008;121:1033-41. [Crossref] [PubMed]
- Fulton JE, Suarez M, Silverton K, et al. Small volume fat transfer. Dermatol Surg 1998;24:857-65. [Crossref] [PubMed]
- Isern AE, Manjer J, Malina J, et al. Risk of recurrence following delayed large flap reconstruction after mastectomy for breast cancer. Br J Surg 2011;98:659-66. [Crossref] [PubMed]
- EORTC Breast Cancer Cooperative Group. EORTC Radiotherapy Group., Bijker N, Meijnen P, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853--a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol 2006;24:3381-7. [Crossref] [PubMed]
- Johansen H, Kaae S, Jensen MB, et al. Extended radical mastectomy versus simple mastectomy followed by radiotherapy in primary breast cancer. A fifty-year follow-up to the Copenhagen Breast Cancer randomised study. Acta Oncol 2008;47:633-8. [Crossref] [PubMed]
- Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991;19:403-10. [Crossref] [PubMed]
- Ghajar CM, Peinado H, Mori H, et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol 2013;15:807-17. [Crossref] [PubMed]
- Cianfrocca M, Goldstein LJ. Prognostic and predictive factors in early-stage breast cancer. Oncologist 2004;9:606-16. [Crossref] [PubMed]
- Saez RA, McGuire WL, Clark GM. Prognostic factors in breast cancer. Semin Surg Oncol 1989;5:102-10. [Crossref] [PubMed]
- Nemoto T, Natarajan N, Bedwani R, et al. Breast cancer in the medial half. Results of 1978 National Survey of the American College of Surgeons. Cancer 1983;51:1333-8. [Crossref] [PubMed]
- Brackstone M, Townson JL, Chambers AF. Tumour dormancy in breast cancer: an update. Breast Cancer Res 2007;9:208. [Crossref] [PubMed]
- Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer 2007;7:834-46. [Crossref] [PubMed]
- Cursiefen C, Chen L, Borges LP, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest 2004;113:1040-50. [Crossref] [PubMed]
- McGuckin MA, Cummings MC, Walsh MD, et al. Occult axillary node metastases in breast cancer: their detection and prognostic significance. Br J Cancer 1996;73:88-95. [Crossref] [PubMed]
- Cote RJ, Peterson HF, Chaiwun B, et al. Role of immunohistochemical detection of lymph-node metastases in management of breast cancer. International Breast Cancer Study Group. Lancet 1999;354:896-900. [Crossref] [PubMed]