Use of acellular dermal matrix (ADM) in nipple reconstruction: the ‘central-pillar technique’
Introduction
A number of techniques for reconstructing the nipple-areolar complex have been developed, but no single method reliably yields a consistent aesthetic result with durable nipple projection. In a retrospective study assessing patient satisfaction in nipple reconstruction, the factor patients disliked most about their nipple reconstruction was the lack of projection (1). The authors present a case series of ten patients undergoing 13 nipple reconstructions with either very thin skin or previously flattened nipple reconstructions. We suggest the use of a nipple shaped cylinder of layered acellular dermal matrix (ADM) as an adjunct to nipple reconstruction to help maintain projection, with a separate piece used to strengthen the platform of de-epithelialised skin on which the reconstruction is supported. Guerra et al. used an arrow flap technique with rib cartilage graft as the internal augmentation (2). The use of rolled ADM as an internal augmentation to maintain projection in nipple reconstructions has previously been demonstrated in an animal model (3) and a series using cylindrical blocks of extracellular, completely absorbable, porcine-derived collagen nipple cylinders has been previously described in humans (4). The use of ADMs in the augmentation of nipple reconstructions was first described by Nahabedian in 2005 (5). This paper seeks to develop the concept.
Technique
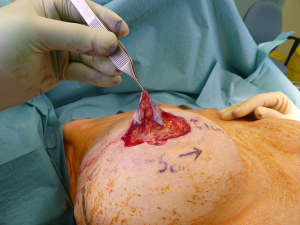
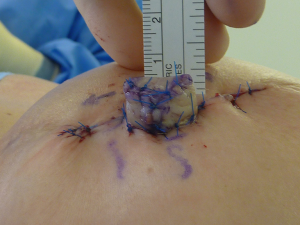
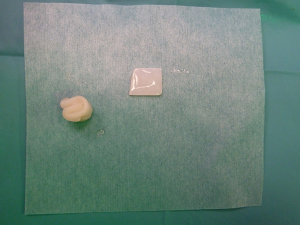
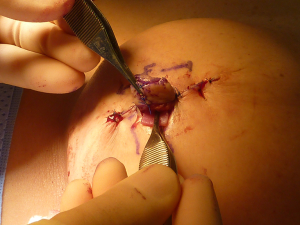
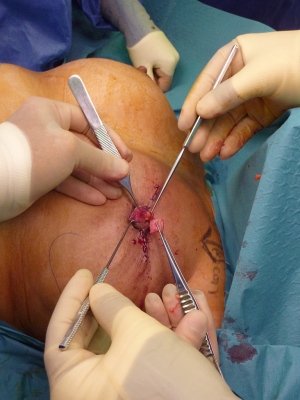
Standard skin flaps for nipple reconstruction are raised. Our usual preference is an arrow flap technique (Figure 1). A 3 cm × 3 cm sheet of 1 mm thick SurgiMend (TEI Biosciences, Boston, Mass.) is divided into three 3 cm × 1 cm rectangles (unit cost £230 excl. VAT). One of the 3 cm × 1 cm rectangles is rolled moderately tightly between the forefinger and thumb with the aid of a Gillies forceps into a “Swiss-roll” tube (Figure 2) and held in place with a 3/0 un-dyed vicryl suture. This forms the central pillar of the nipple reconstruction. A separate 3 cm × 1 cm strip is divided into three 1 cm × 1 cm squares (Figure 2). Adequate ADM is available for a bilateral case by rolling the second of the 3 cm × 1 cm strips that is used in conjunction with a further 1 cm × 1 cm square. In the arrow technique, a de-epithelialized disc forms the base on which the raised nipple reconstruction rests, reducing the possibility of the nipple falling into the space created by raising the flap. The 1 cm × 1 cm square acts as a platform and is secured onto the de-epithelialised base with 3-0 polydioxanone sutures (Figure 3). The rolled cylinder of ADM is then supported by positioning it onto this base to allow it to act as the central pillar of the re-created nipple (Figure 4). The wings of the raised nipple flap are then sutured around the central pillar. The partial thickness skin flap originating from the disc that forms the base of the nipple is used to close the top of the nipple reconstructions (Figure 5).
Patients and methods
Data was collected prospectively on a consecutive series by a single surgeon including patient demographics, previous procedures and adjuvant therapies. Nipple projection and diameter was measured using callipers at the end of the procedure, 6 months post-operatively and 12 months post-operatively. Inclusion criteria for use of the technique were either primary patients noted to have exceptionally thin dermis over the reconstructed breast or revision cases of primary reconstructions that had flattened.
Results
The technique was used for 13 reconstructions in ten patients. Six reconstructions in 4 patients (2 bilateral cases) were primary procedures in patients with very thin dermis. Seven reconstructions in 6 patients (1 bilateral case) were for secondary revisions of flattened primary reconstructions (Figure 6). Five patients had ADM assisted implant-based reconstructions, 3 patients had pedicled latissimus dorsi myocutaneous flap reconstructions with implants and 1 patient had a central wide local excision including sacrifice of the nipple-areolar complex. Two patients had previous chemotherapy and 1 patient had previous radiotherapy.

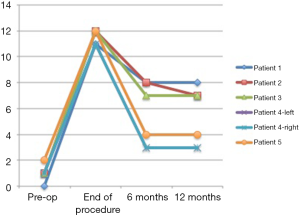
Of the primary procedures in patients with thin dermis the average nipple projection at the end of the procedure was 10.2 mm and at 12 months post-operative was 5.2 mm, demonstrating a 51% preservation of nipple height (Figure 7). Nipple diameter was 10.2 mm at the end of the procedure and 9.2 mm at 12 months demonstrating a 90% preservation of diameter.
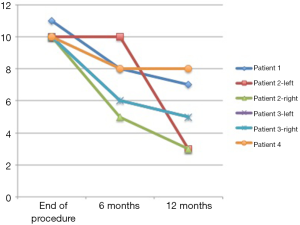
Of the secondary nipple reconstructions for revision of a previous procedure that had completely flattened the average nipple projection at the end of the procedure was 11.5 mm and at 12 months post-operative was 5.3 mm (Figure 8). This represents a similar 46% preservation of nipple projection. Average diameter was 11.5 mm at the end of the procedure and 9.2 mm at 12 months showing 80% preservation.
Twenty percent patients (3/13 reconstructions) had a loss of nipple projection of greater than 60% and required a further procedure. Both of these were in the revision group. No patients had either partial or complete extrusion of the ADM or any other complications. One patient in the primary group had loss of 70% projection in bilateral reconstructions, but was happy with the result and did not require further revision. The other reconstructions maintained an average of over 60% projection at 12 months.
Discussion
There have been a number of review articles discussing the advantages and disadvantages of different nipple reconstruction techniques (6-9). However, loss of projection in nipple reconstructions remains a common problem that is often difficult to predict. Patients with very thin skin often prove the most difficult and give the least predictable results particularly if they have large contralateral nipples. In our experience, patients who have already had a failed reconstruction are also at high risk of flattening again.
Several methods have previously been described to augment nipple reconstructions and reduce or treat loss of projection. These have included the use of numerous autologous materials such as rib cartilage (2), auricular cartilage (10), dermal grafts (11) and fat grafts (12). Many of these materials involve introducing a donor site for graft harvest. Nipple reconstructions are usually local anaesthetic procedures in skin with reduced sensation and are thus exceptionally well tolerated by patients. The use of these extra donor sites can be painful, less well tolerated, require longer procedures and sometimes general anaesthesia. They also tend to have more variable results and higher complication rates so are now largely of historical interest only.
Non-autologous methods of nipple augmentation have included the use of artificial bone (13), polyurethane coated silicone (14) and the injection of polymethylmethacrylate microspheres suspended in bovine collagen (15). However, the clinical experience of many surgeons who have used synthetic products have been variable with problems of extrusion perhaps being most common.
One group that used synthetic collagen cylinders derived from porcine small bowel submucosa reported an extrusion rate of 5% (4). Nahabedian first described the use of a folded piece of human derived ADMs (AlloDerm, LifeCell Corp., Branchburg, NJ, USA) with C-V flaps for four patients with previous flattening needing secondary nipple reconstructions (5). Rolled Alloderm nipple reconstructions were then described with skate flaps in primary reconstructions with maintenance of projection of 47–56% (16). A more recent paper suggested a variety of ADM constructs using a human derived product made in Korea (MegaDerm, L&C Bio Corp., Seoul, Korea) in primary nipple reconstructions; depending on the type of underlying breast reconstruction, the authors reported average maintenance of projection of around 70% at 9 months (17).
Other reconstructive techniques have mainly involved composite grafts from elsewhere as the whole nipple reconstruction. This has included nipple sharing procedures (18,19) and the grafting of free toe pulps (20,21). Nipple sharing procedures are sometimes popular in women with a lot of natural projection on one side who are happy to sacrifice a degree of function on the donor site to create two nipples with half as much projection. The use of toe pulps are now of historical interest only.
Our results presented here suggest that nipple reconstructions with the foetal bovine-derived ADM SurgiMend (TEI Biosciences, Boston, Mass.) achieve comparable results to human-derived alternatives previously (5,16,17,22). The extra expense of using a small piece of ADM appears to be justified by the comparative reliable maintenance of both projection and diameter of the reconstructions in patients. The patients included in this study were at greater risk than usual of flattening to a degree that would otherwise require a further procedure. A 3 cm × 3 cm piece is sufficient for two reconstructions so no extra cost is incurred for bilateral cases. There were no other complications in this series. The technique also appears safe in radiotherapy fields and has similar results when used in thin mastectomy tissue directly over an implant compared to thicker autologous tissue over a smaller implant. This technique was not used in purely autologous reconstructions, but is likely to have similar outcomes. The only disadvantages of using ADMs in this setting are the modest cost implications, which we feel are warranted, and the theoretical increased risks of either flap necrosis or implant extrusion, which was not seen in our series.
Our results suggest that the ADM central pillar helps to maintain long-term projection as a relatively solid block without being reabsorbed in nipple reconstruction. The de-epithelialised dermal base, reinforced with the 1 cm × 1 cm sheet of ADM, also appears to help prevent late retraction back into the subcutaneous tissue. A randomized controlled trial would provide direct comparison of long-term projection to a control group without ADM augmentation.
Acknowledgements
The Royal Marsden is an NIHR Biomedical Research Centre and this support is acknowledged. The authors are also grateful to TEI Biosciences for a research stipend that part funded this study.
Footnote
Conflicts of Interest: These authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Jabor MA, Shayani P, Collins DR Jr, et al. Nipple-areola reconstruction: satisfaction and clinical determinants. Plast Reconstr Surg 2002;110:457-63; discussion 464-5. [Crossref] [PubMed]
- Guerra AB, Khoobehi K, Metzinger SE, et al. New technique for nipple areola reconstruction: arrow flap and rib cartilage graft for long-lasting nipple projection. Ann Plast Surg 2003;50:31-7. [Crossref] [PubMed]
- Holton LH, Haerian H, Silverman RP, et al. Improving long-term projection in nipple reconstruction using human acellular dermal matrix: an animal model. Ann Plast Surg 2005;55:304-9. [Crossref] [PubMed]
- Tierney BP, Hodde JP, Changkuon DI. Biologic collagen cylinder with skate flap technique for nipple reconstruction. Plast Surg Int 2014;2014:194087. [Crossref] [PubMed]
- Nahabedian MY. Secondary nipple reconstruction using local flaps and AlloDerm. Plast Reconstr Surg 2005;115:2056-61. [Crossref] [PubMed]
- Sisti A, Grimaldi L, Tassinari J, et al. Nipple-areola complex reconstruction techniques: A literature review. Eur J Surg Oncol 2016;42:441-65. [Crossref] [PubMed]
- Nimboriboonporn A, Chuthapisith S. Nipple-areola complex reconstruction. Gland Surg 2014;3:35-42. [PubMed]
- Winocour S, Saksena A, Oh C, et al. A Systematic Review of Comparison of Autologous, Allogeneic, and Synthetic Augmentation Grafts in Nipple Reconstruction. Plast Reconstr Surg 2016;137:14e-23e. [Crossref] [PubMed]
- Farhadi J, Maksvytyte GK, Schaefer DJ, et al. Reconstruction of the nipple-areola complex: an update. J Plast Reconstr Aesthet Surg 2006;59:40-53. [Crossref] [PubMed]
- Tanabe HY, Tai Y, Kiyokawa K, et al. Nipple-areola reconstruction with a dermal-fat flap and rolled auricular cartilage. Plast Reconstr Surg 1997;100:431-8. [Crossref] [PubMed]
- Eo S, Kim SS, Da Lio AL. Nipple reconstruction with C-v flap using dermofat graft. Ann Plast Surg 2007;58:137-40. [Crossref] [PubMed]
- Bernard RW, Beran SJ. Autologous fat graft in nipple reconstruction. Plast Reconstr Surg 2003;112:964-8. [Crossref] [PubMed]
- Yanaga H. Nipple-areola reconstruction with a dermal-fat flap: technical improvement from rolled auricular cartilage to artificial bone. Plast Reconstr Surg 2003;112:1863-9. [Crossref] [PubMed]
- Hallock GG. Polyurethane nipple prosthesis. Ann Plast Surg 1990;24:80-5. [Crossref] [PubMed]
- McCarthy CM, VanLaeken N, Lennox P, et al. The efficacy of Artecoll injections for the augmentation of nipple projection in breast reconstruction. Eplasty 2010;10:e7. [PubMed]
- Garramone CE, Lam B. Use of AlloDerm in primary nipple reconstruction to improve long-term nipple projection. Plast Reconstr Surg 2007;119:1663-8. [Crossref] [PubMed]
- Park GY, Yoon ES, Cho HE, et al. Acellular Dermal Matrix as a Core Strut for Projection in Nipple Reconstruction: Approaches for Three Different Methods of Breast Reconstruction. Arch Plast Surg 2016;43:424-9. [Crossref] [PubMed]
- Millard DR Jr. Nipple and areola reconstruction by split-skin graft from the normal side. Plast Reconstr Surg 1972;50:350-3. [Crossref] [PubMed]
- Bhatty MA, Berry RB. Nipple-areola reconstruction by tattooing and nipple sharing. Br J Plast Surg 1997;50:331-4. [Crossref] [PubMed]
- Klatsky SA, Manson PN. Toe pulp free grafts in nipple reconstruction. Plast Reconstr Surg 1981;68:245-8. [Crossref] [PubMed]
- Amarante JT, Santa-Comba A, Reis J, et al. Halux pulp composite graft in nipple reconstruction. Aesthetic Plast Surg 1994;18:299-300. [Crossref] [PubMed]
- Chen WF, Barounis D, Kalimuthu R. A novel cost-saving approach to the use of acellular dermal matrix (AlloDerm) in postmastectomy breast and nipple reconstructions. Plast Reconstr Surg 2010;125:479-81. [Crossref] [PubMed]