Estimation of tumor size in breast cancer comparing clinical examination, mammography, ultrasound and MRI—correlation with the pathological analysis of the surgical specimen
Introduction
Estimation of tumor size is basic in order to decide the type of treatment in breast cancer: mastectomy or lumpectomy, orif it is necessary to start with neoadjuvant chemotherapy (NAC). For decision making in clinical practice we rely on clinical examination and imaging tests, which are mammography, magnetic resonance imaging (MRI) and ultrasound (US). Mammography has always been considered the gold standard for diagnosis and to know the tumor size, but in recent years high resolution US and MRI have been strongly incorporated. These imaging techniques inform about the size of the lesion in order to choose the best treatment for the patient, always taking into account that the size of the lesion is given in the final pathologic examination (surgical specimen in millimeters).
Mammography has the disadvantage of being less accurate in high density breast (young age), but it is very useful in patients with microcalcifications (1). The use of digital mammography and 3D tomosynthesis mammogram has greatly improved the sensitivity of these techniques (2). MRI has a high sensitivity but low specificity, as it tends to overestimate lesion size, but it is very useful to discover multicentricity (3,4).
High definition US is a not expensive and it is also a simple technique, but it is operator dependent. It has the advantage of detecting non-palpable lesions in the operating room avoiding the use of wires or radioguided occult lesion localization (ROLL).
There are numerous studies comparing mammography, US and MRI between them and separately, with disparate results. Some studies report that US is better than MG (4,5). Other studies report that MRI is superior to that of the MG and the US (6,7).
We do not want to check which the best diagnostic method is. The aim of this study is to compare tumor size estimated by clinical examination and new imaging techniques (3D tomosynthesis mammography, US high resolution, MRI) with the tumor size (in millimeters) in surgical specimen, in order to assess which is the most accurate method to know the “true” tumor size.
Methods
We retrospectively collected cases of breast cancer from November 2015 to July 2016 in our center. All patients were diagnosed using Core-biopsy. The mean age and histological type of tumor in all patients is recorded. Ductal carcinoma in situ (DCIS) and patients who underwent NAC were excluded.
All patients were studied by: clinical examination performed by senology expert gynecologist or surgeon, with measure ruler, mammography 3D tomosynthesis, high resolution US (General Electric LogiQ9), and MRI. Surgical specimens were studied by the same pathologist.
Correlation with tumor size was studied calculating Pearson correlation index. A Wilcoxon signed-rank test was applied to measure differences between values obtained with each of the techniques versus real tumor size. The concordance correlation (Lin, 1989) coefficient is a useful index to evaluate precision and accuracy of different diagnosis techniques. It adds a bias correction to the Pearson coefficient that measures how far the best-fit line deviates from the 45° line through the origin. Lin’s index was calculated for each of the techniques. Level of significance was set at 95% (P=0.05). R studio software was used for statistical calculations (RStudio v.0.99.879: integrated development environment for R. R studio, Inc., Boston, MA, USA 2015).
Results
A total of 73 cases were collected from October 2015 to July 2016 with diagnosis of invasive breast carcinoma. Twelve cases of DCIS and seven patients who underwent NAC were excluded. We also excluded three cases with positive margins. Finally, a total of 56 cases were included in the analysis. Excision of the lesions was guided either by US (even if palpable), wire or ROLL. The mean age of the patients was 57 years.
Histological types were invasive ductal carcinoma (IDC) in 46 patients (80.7%), invasive lobular carcinoma (ILC) in 8 (14%), and other carcinomas in 3 cases (5.2%).
When comparing mean values in each of the diagnostic techniques versus tumor size (16 and 10 mm), a significant higher value was observed with MRI (22 and 53 mm). No significant differences were observed with clinical exploration, US and mammography, 12,17; 14,5 and 14,32 respectively (Table 1).
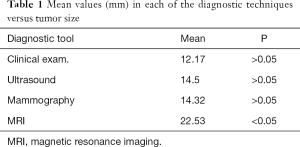
Full table
All diagnostic techniques showed a significant association with tumor size when Pearson coefficient was calculated.
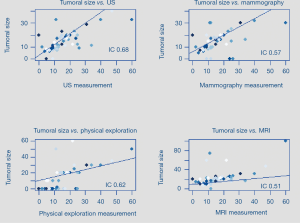
Pearson correlation results were the following: for the physical exam 0.62 (0.43–0.76; 95% CI), for US examination 0.68 (0.51–0.8; 95% CI), for mammography 0.57 (0.36–0.72; 95% CI), and for MRI 0.51 (0.29–0.68; 95% CI), compared to surgical specimen examination by pathologist (Figure 1).
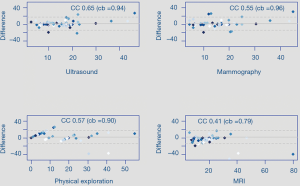
Lin’s index was 0.65 for US and 0,55; 0,57 and 0,41 for mammography, physical exam and MRI respectively, indicating that sonography was more precise than the other techniques (Table 2).

Full table
Bland-Altman plots shows distribution of differences observed with each diagnostic technique compared with infiltrative component of biopsies (Figure 2).
Discussion
There are several studies that compare the accuracy of MM, US and MRI to predict tumor size (3-11). These studies are summarized in Table 3.
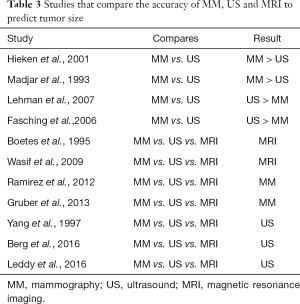
Full table
In one of the most recent study, Leddy et al. (9) consider that the MRI overestimates tumor size and measurements obtained with US and MM are more accurate independently of breast density, and the US was superior to MM to predict the tumor size.
In our study US was considered the best predictor of tumor size in breast cancer, comparing with clinical examination, mammography, and MRI. We also observed that MRI significantly overestimated the lesion size. These results are congruent with other studies published in the literature (6-9,12,13).
Breast density had a negative influence on the sensitivity of mammography, with sensitivity of 30–48% in very dense breast (10,14-16). Our study compares the three techniques in all patients, we do not use analogic or digital MM, we use Mammography3D tomosynthesis, maybe can explain why we visualized the tumor by MM in all of our patients independently of mammary density, since in other studies MM does not always visualize the tumor when using analogic or digital MM (5,6,12,15-17). However, mammography continues to be a gold standard test, since it allows visualization of lesions such as microcalcifications or in situ carcinoma, in which US has a low utility.
The use of MRI has been implemented in all breast units, and in many centers it is practiced on a regular basis. MRI has a very high sensitivity (90–99%) but low specificity. Houssami et al. (14) report that Resonance overestimates size, performing more mastectomies than necessary, making the process more expensive, since its low specificity makes it necessary to perform “2nd US look” and more biopsies. Behjatnia et al. (18) reported that MRI overestimated the size in 70% of their patients. Leddy et al. (9) also report that MRI overestimates the size of the lesion compared to the surgical specimen.
Ultrasonography, in our study, is the most accurate method when compared to the tumor size of the surgical specimen. It is a simple, noninvasive and fast technique, but it is operator dependant. Our study has been performed in a single institution, tests have been performed by the same radiologists and surgeons, and surgical pieces have been studied by the same pathologist.
In our series only 14% were ILC. It is known that ILC, due to its diffuse histological growth through collagen, is more difficult to evaluate by US and mammography (19,20), but in our study we did not see a worse correlation. In these histological subtypes the MRI could play a more important role. Leddy et al., report that MRI underestimates the tumor size of the ILC in 78% of the cases.
In breast cancer, it is necessary to individualize each case, since depending on the biology of the tumor and other factors the estimation of tumor size by US can vary, and we must take into account all methods of pre-surgical study, using clinical examination, mammography, US and MRI as complementary tests, knowing the strengths and weaknesses of each test, in order to plan the best treatment for the patient.
Our study was retrospective and with a small number of patients, but we observed that in patients with a unifocal tumor, MM and US provided a more accurate estimation of tumor size than the MRI, which overestimated it. These results have to be taken judiciously, as we need more prospective studies with larger series. Our work could help in the decision-making process such as the type of conservative surgery, the possible need for oncoplastic surgery or the decision to start treatment with neoadjuvant therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Due to the retrospective nature of this study, the Institutional Review Board has exempted the ethics approval.
References
- Feig SA. Breast masses. Mammographic and sonographic evaluation. Radiol Clin North Am 1992;30:67-92. [PubMed]
- Pisano ED, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med 2005;353:1773-83. [Crossref] [PubMed]
- Lehman CD, Gatsonis C, Kuhl CK, et al. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med 2007;356:1295-303. [Crossref] [PubMed]
- Hieken TJ, Harrison J, Herreros J, et al. Correlating sonography, mammography, and pathology in the assessment of breast cancer size. Am J Surg 2001;182:351-4. [Crossref] [PubMed]
- Madjar H, Ladner HA, Sauerbrei W, et al. Preoperative staging of breast cancer by palpation, mammography and high-resolution ultrasound. Ultrasound Obstet Gynecol 1993;3:185-90. [Crossref] [PubMed]
- Boetes C, Mus RD, Holland R, et al. Breast tumors: comparative accuracy of MR imaging relative to mammography and US for demonstrating extent. Radiology 1995;197:743-7. [Crossref] [PubMed]
- Wasif N, Garreau J, Terando A, et al. MRI versus ultrasonography and mammography for preoperative assessment of breast cancer. Am Surg 2009;75:970-5. [PubMed]
- Ramirez SI, Scholle M, Buckmaster J, et al. Breast cancer tumor size assessment with mammography, ultrasonography, and magnetic resonance imaging at a community based multidisciplinary breast center. Am Surg 2012;78:440-6. [PubMed]
- Leddy R, Irshad A, Metcalfe A, et al. Comparative accuracy of preoperative tumor size assessment on mammography, sonography, and MRI: Is the accuracy affected by breast density or cancer subtype? J Clin Ultrasound 2016;44:17-25. [Crossref] [PubMed]
- Gruber IV, Rueckert M, Kagan KO, et al. Measurement of tumour size with mammography, sonography and magnetic resonance imaging as compared to histological tumour size in primary breast cancer. BMC Cancer 2013;13:328. [Crossref] [PubMed]
- Fasching PA, Heusinger K, Loehberg CR, et al. Influence of mammographic density on the diagnostic accuracy of tumor size assessment and association with breast cancer tumor characteristics. Eur J Radiol 2006;60:398-404. [Crossref] [PubMed]
- Yang WT, Lam WW, Cheung H, et al. Sonographic, magnetic resonance imaging, and mammographic assessments of preoperative size of breast cancer. J Ultrasound Med 1997;16:791-7. [Crossref] [PubMed]
- Berg WA, Gutierrez L. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology 2004;233:830-49. [Crossref] [PubMed]
- Houssami N, Turner R, Morrow M. Preoperative magnetic resonance imaging in breast cancer: meta-analysis of surgical outcomes. Ann Surg 2013;257:249-55. [Crossref] [PubMed]
- Fornage BD, Toubas O, Morel M. Clinical, mammographic, and sonographic determination of preoperative breast cancer size. Cancer 1987;60:765-71. [Crossref] [PubMed]
- Heusinger K, Löhberg C, Lux MP, et al. Assessment of breast cancer tumor size depends on method, histopathology and tumor size itself*. Breast Cancer Res Treat 2005;94:17-23. [Crossref] [PubMed]
- Shin HC, Han W, Moon HG, et al. Limited value and utility of breast MRI in patients undergoing breast-conserving cancer surgery. Ann Surg Oncol 2012;19:2572-9. [Crossref] [PubMed]
- Behjatnia B, Sim J, Bassett LW, et al. Does size matter? Comparison study between MRI, gross, and microscopic tumor sizes in breast cancer in lumpectomy specimens. Int J Clin Exp Pathol 2010;3:303-9. [PubMed]
- Lopez JK, Bassett LW. Invasive lobular carcinoma of the breast: spectrum of mammographic, US, and MR imaging findings. Radiographics 2009;29:165-76. [Crossref] [PubMed]
- Arpino G, Bardou VJ, Clark GM, et al. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res 2004;6:R149-56. [Crossref] [PubMed]


