Usefulness of CBCT and guidance software for percutaneous embolization of a lymphatic leakage after thyroidectomy for cancer
Introduction
Chylous leak is a rare complication after thyroid surgery. The risk of lymphatic leakage increases in case of thyroid surgery associated with neck lymphadenectomy: 0.5% risk after subtotal thyroidectomy and central compartment lymph node dissection; 0.8% after total thyroidectomy and central compartment lymph node dissection, 5.1% after total thyroidectomy and lateral lymph node dissection on one side, 6.2% after total thyroidectomy and bilateral lymph node dissection) (1-3).
In case of thoracic duct leakage during thyroidectomy chylothorax can occur. Two mechanisms for iatrogenic chylothorax are reported in the literature (4): a direct injury during surgery could cause a chyle drain from the base of the neck into the mediastinum or ligation of thoracic duct could produce an increase of backward hydrostatic pressure causing an extravasation of chyle or a secondary rupture of the thoracic duct. In both cases, there is no direct injury to the pleura (explaining the absence of pneumothoraces), the chyle penetrates into the pleura as a result of tissue maceration (1-3).
The risk factors for chylothorax in thyroid surgery include: advanced thyroid cancer, left side lymphadenectomy, massive goiter and intrathoracic localization. A recent review of literature reported an incidence of chylothorax in thyroidectomy with cervical approach of 1.85% (4).
Chyle is composed primarily of fat and digestive products absorbed by the intestinal lacteal system. Chyle is bacteriostatic and is a significant source of immuno- globulins and T lymphocytes, with concentrations ranging from 400 to 6,800 cells/mL (5). Persistent high-output chylothorax can result in life-threatening malnutrition, susceptibility to infection, and metabolic derangements (6). Conservative management succeeded in only a minority of cases (27.7%) (7), the majority of patients require surgery. Surgical management can be technically difficult due to the high incidence of variant anatomy and the high-risk patient population (8). In 1996, Cope et al. (9) described thoracic duct embolization (TDE) and thoracic duct disruption (TDD) as minimally invasive treatments for chylous effusion. Since its description, several case reports and case series have been published (10), describing TDE, TDD and peripheric lymphatic leak embolization. Two series were published, respectively with 42 (10) and 109 (11) patients reporting a response rate of 73.8% and 71% respectively, with a low complication rate (3%).
We report a case of refractory cervical chylous leakage after left radical neck dissection that was successfully treated with an ultrasound-guided intranodal lymphangiography and a percutaneous puncture of the leak performed using CBCT as imaging guidance.
Case presentation
A 46-year-old Caucasian men presented at our Institution with a self-detected left thyroidal nodule. His medical history was unremarkable. On the basis of the rapid dimensional increase and of the ultrasound findings, fine needle ago-biopsy was performed, resulting in medullary thyroidal carcinoma. Staging was performed with a neck and thorax Contrast Enhanced CT (CECT) scan, resulting in localised disease. The Patient underwent a total thyroidectomy and radical neck dissection with intraoperative neuro-monitoring of recurrent laryngeal nerve. The operation proceeded without any complication and recurrent laryngeal nerves were successfully identified and preserved. The histologic diagnosis reported a pT2 pN0 medullary thyroidal carcinoma. Left parathyroid gland was not identified and was included in histology. On the evening of the 1st postoperative day, a milky and turbid fluid was found in his surgical drainage. A lymphatic leakage was suspected. The fluid was sent for biochemical analysis and a significantly raised triglyceride level of 187 mg/dL served to confirm its chylous nature.
The patient was immediately undergone to total parenteral nutrition (TPN) with lipid emulsions, pressure dressings, octreotide somministration and lower-pressure suction drainage. On postoperative day 6, in light of persistent chylous leakage (>2,000 mL), the case was presented to a multidisciplinary team decision and interventional radiology procedure was proposed. Our Internal Review Board approved the procedure. Written informed consent was obtained from the patient.
Procedure
The procedure was performed in our Angiosuite, equipped with a CBCT C-arm angiograph (Allura Xper FD20 with flat detector, Philips Medical Systems, Best, The Netherlands) with a dedicated workstation running guidance and volumetric planning software (XperGuide System, Philips Medical Systems).
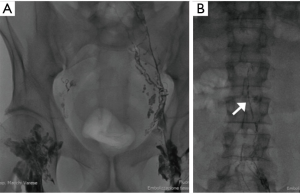
Bilateral inguinal nodes were directly accessed under ultrasound guidance (Philips iU22, Best, The Netherlands) with a 20-gauge spinal needle (Biopsybell, Medical Devices, Modena, Italy). The needle tip was positioned in the transitional zone between the cortex and hilum of the lymph node. Under fluoroscopic guidance, lipophilic contrast media (ethiodized oil: Lipiodol®, Guerbert, Villepinte, France) was injected by hand at a rate of about 1 mL per 5 minutes. A total volume of approximately 3–6 mL of lipiodol was injected into each lymph node. Initial injection was observed under fluoroscopic guidance to confirm proper positioning of the needle and to identify the efferent lymphatic (Figure 1A,B).
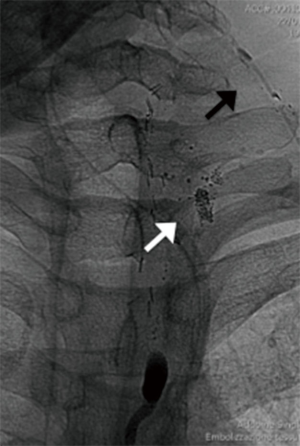
Serial fluoroscopic controls were performed (about every 30 minutes) to monitor the progression of the opacification of the lymphatic system. Cisterna chyli and thoracic duct were identified after 2 and 3 h respectively. After 3 h and 30 minutes a contrast blush was appreciated on the left side of the neck, close to the apex of the surgical drainage (Figure 2).
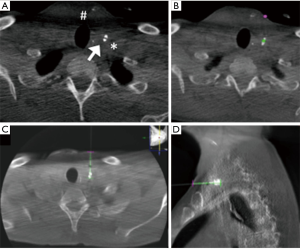
The puncture of the leak was performed under CBCT guidance using XperGuide software. A CBCT acquisition was performed using 620 rotational fluoroscopic-like frames with a fast motion of the ‘‘C’’ rotating arm. The reconstruction was obtained using an isotropic volume (matrix 3,843 voxel size of 0.20–0.49 mm according to the initial set-up of the field of view) obtained on the same workstation. On the basis of this acquisition, we chose the shortest route from the skin to the leak, avoiding vascular structures in the needle path. The trajectory was automatically calculated by the system in all the planes on the basis of the entry point and target point indicated by the operator (Figure 3). CBCT and real-time fluoroscopic images were automatically merged and the C-arm identified two different views: the ‘‘entry point’’ (in which entrance and target were overlapped) and ‘‘progression view’’ (perpendicular to the entry point view). Xper-Guide software is automatically able to calculate the correct position of the angiographic C-arm to display the ‘‘entry point’’ of the needle on the patient’s skin. Under fluoroscopic guidance, the operator positioned the tip of the needle (20G ×15 cm, Biopsybell, Medical Devices, Modena, Italy) on the patient’s skin at the ‘‘entry point’’. The needle was advanced to the target point using the progression view, perpendicular to the preceding one. It represents a virtual path previously determined and displayed in real time, under fluoroscopy on the dedicated monitor. A second C-arm CBCT scan was performed to confirm whether the tip was located within the leak (Figure 4). Embolization was performed with the injection of 0.2 mL Glubran II (Glubran 2, N-butyl-2-cyanoacrylate; GEM S.r.l., Viareggio, Italy) (with a diluition of 1:1).
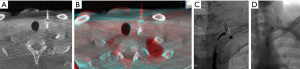
Before the beginning of the procedure, local anesthesia of the access site was achieved with a subcutaneous injection of a 10-mL solution of 2% Mepivacaine (Mepivacaina, 2%, Angelini SpA, Rome, Italy). Heart rate, electrocardiographic trace, oxygen saturation, respiratory frequency, and blood pressure were continuously monitored throughout the procedure.
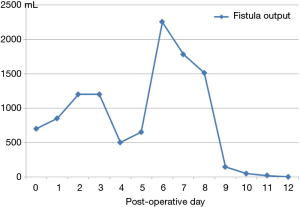
The patient tolerated the procedure well without any minor or major adverse events. Clinical success was determined by measurement of the daily tube outputs the day before, day of, and each day for 4 days after the procedure. The tube was removed when out-put was less than 20 mL/day (Figure 5). The patient was discharged after 12 days. To date, with a follow up of 30 months, neither recurrences nor sequelae related to the surgical complication or the interventional procedure were registered.
Discussion
Chylous leakage is a rare complication of head and neck surgery. Correct management can be problematic and prolonged. They will frequently close spontaneously, consequently the initial management is conservative and consists of alleviation of symptoms, replacement of fluid and nutrient losses and reduction of chyle output to facilitate spontaneous healing (8). Somatostatin is an inhibitor of gastrointestinal secretions that helps to keep the gastrointestinal tract empty, thereby reducing chyle production. The duration for which conservative therapy should be trialed has not been firmly established. Surgical management of peripheric lymphatic leakages can be problematic; failure to identify the thoracic duct (due to anatomical variations, intervening pathologies, and other causes) and failures in ligation and occlusion of the defect must be considered and make the intervention inconclusive (8). Percutaneous, image-guided techniques may offer two advantages: mini-invasivity and ability to image and identify the anatomy and the site of the leakage.
Cope reported the first successful percutaneous treatment of chyle leaks in 1998 and 1999 (12,13). Lymphangiography is necessary to opacify a target for embolization.
Conventional pedal lymphangiography is a technically challenging procedure that requires isolation and cannulation of pedal lymphatic vessels followed by infusion of oily contrast material over a period of hours (14). A novel intranodal lymphangiography by which blind injection of lipiodol is performed from the groin lymph nodes is said to be less difficult and time-consuming (15,16).
Several reasons led us to apply the technique described by Nadolski et al. (16), in particular they suggested the puncture of the junction of the cortex and hilum of the lymph node to allow sufficient contrast agent uptake and minimize extravasation; moreover, the use of a fine needle helps to limit the rate of injection. Intranodal lymphangiogram seems to reduce the time needed to visualize the thoracic duct and consequently the total procedure time.
The described case emphasizes the use of CBCT and its guidance planning software (XperGuide System, Philips Medical Systems). Fluoroscopy documented the blush of lipophilic contrast media next to the tip of the tube of drainage. On the basis of our experience and compliance with CBCT and its applications during interventional procedures (17-19), we decided to use it as imaging guidance for the puncture of the leakage. In fact, ultrasound guidance can identify the important anatomical structures (e.g., carotid artery or laryngeal nerves) but cannot clearly identify leakage site. On the other hand, fluoroscopic guidance can identify leakage site but cannot identify thorny structure. CBCT seemed to be an accurate and immediate tool to asses and treat leaking lymphatics, thanks to the combination of fluoroscopic and tomographic imaging modalities.
Moreover, CBCT equipped with its guidance software, permits to identify a safe live 3D needle path avoiding the puncture of vital structures, overlapping fluoroscopy and acquired CBCT images. In our case the procedure was safe and efficacious. We hypothesized the success of the procedure both to the embolization with glue than to the needle disruption technique (8). In literature, the latter technique is described when major lymphatics causing leakage are too small or numerous to cannulate. The mechanism by which needle disruption decreases chyle leakage is unknown. One hypothesis is that it slows down the lymphatic flow, allowing the body to seal the leak (6).
In our knowledge, our case represents the first case of percutaneous embolization of a peripheric lymphatic leakage performed using CBCT as imaging guidance. In conclusion, in a patient with challenging and persistent lymphatic leak, percutaneous treatment may be considered and CBCT represents a valid and safe tool to guide puncture and embolization.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this Case report and any accompanying images.
References
- de Gier HH, Balm AJ, Bruning PF, et al. Systematic approach to the treatment of chylous leakage after neck dissection. Head Neck 1996;18:347-51. [Crossref] [PubMed]
- Gregor RT. Management of chyle fistulization in association with neck dissection. Otolaryngol Head Neck Surg 2000;122:434-9. [PubMed]
- Lee YS, Nam KH, Chung WY, et al. Postoperative complications of thyroid cancer in a single center experience. J Korean Med Sci 2010;25:541-5. [Crossref] [PubMed]
- Merki V, Pichler J, Giger R, et al. Chylothorax in thyroid surgery: a very rare case and systematic review of the literature. J Otolaryngol Head Neck Surg 2016;45:52. [Crossref] [PubMed]
- Patel N, Lewandowski RJ, Bove M, et al. Thoracic duct embolization: a new treatment for massive leak after neck dissection. Laryngoscope 2008;118:680-3. [Crossref] [PubMed]
- Binkert CA, Yucel EK, Davison BD, et al. Percutaneous treatment of high-output chylothorax with embolization or needle disruption technique. J Vasc Interv Radiol 2005;16:1257-62. [Crossref] [PubMed]
- Cerfolio RJ, Allen MS, Deschamps C, et al. Postoperative chylothorax. J Thorac Cardiovasc Surg 1996;112:1361-5; discussion 1365-6. [Crossref] [PubMed]
- Lyon S, Mott N, Koukounaras J, et al. Role of interventional radiology in the management of chylothorax: a review of the current management of high output chylothorax. Cardiovasc Intervent Radiol 2013;36:599-607. [Crossref] [PubMed]
- Cope C. Percutaneous transabdominal embolization of thoracic duct lacerations in animals. J Vasc Interv Radiol 1996;7:725-31. [Crossref] [PubMed]
- Cope C, Kaiser LR. Management of unremitting chylothorax by percutaneous embolization and blockage of retroperitoneal lymphatic vessels in 42 patients. J Vasc Interv Radiol 2002;13:1139-48. [Crossref] [PubMed]
- Itkin M, Kucharczuk JC, Kwak A, et al. Nonoperative thoracic duct embolization for traumatic thoracic duct leak: experience in 109 patients. J Thorac Cardiovasc Surg 2010;139:584-89; discussion 589-90. [Crossref] [PubMed]
- Cope C. Diagnosis and treatment of postoperative chyle leakage via percutaneous transabdominal catheterization of the cisterna chyli: a preliminary study. J Vasc Interv Radiol 1998;9:727-34. [Crossref] [PubMed]
- Cope C, Salem R, Kaiser LR. Management of chylothorax by percutaneous catheterization and embolization of the thoracic duct: prospective trial. J Vasc Interv Radiol 1999;10:1248-54. [Crossref] [PubMed]
- Parvinian A, Mohan GC, Gaba RC, et al. Ultrasound-guided intranodal lymphangiography followed by thoracic duct embolization for treatment of postoperative bilateral chylothorax. Head Neck 2014;36:E21-4. [Crossref] [PubMed]
- Rajebi MR, Chaudry G, Padua HM, et al. Intranodal lymphangiography: feasibility and preliminary experience in children. J Vasc Interv Radiol 2011;22:1300-5. [Crossref] [PubMed]
- Nadolski GJ, Itkin M. Feasibility of ultrasound-guided intranodal lymphangiogram for thoracic duct embolization. J Vasc Interv Radiol 2012;23:613-6. [Crossref] [PubMed]
- Floridi C, Muollo A, Fontana F, et al. C-arm cone-beam computed tomography needle path overlay for percutaneous biopsy of pulmonary nodules. Radiol Med 2014;119:820-7. [Crossref] [PubMed]
- Ierardi AM, Petrillo M, Xhepa G, et al. Cone beam computed tomography images fusion in predicting lung ablation volumes: a feasibility study. Acta Radiol 2016;57:188-96. [Crossref] [PubMed]
- Carrafiello G, Fontana F, Mangini M, et al. Initial experience with percutaneous biopsies of bone lesions using XperGuide cone-beam CT (CBCT): technical note. Radiol Med 2012;117:1386-97. [Crossref] [PubMed]


