Is vascular flow a predictor of malignant thyroid nodules? A meta-analysis
Introduction
The incidence of thyroid cancer in the United States has demonstrated a 2.4-fold increase over the last 30 years (1). In 2013, the National Cancer Institute estimated the incidence of thyroid cancer in the United States to be greater than 60,000 new cases (2). Despite the rise in thyroid cancer, current diagnostic abilities for determining malignancy are lacking, and it is estimated that only 1 in 20 thyroid nodules operated on are found to be malignant (3). The superiority of ultrasound (US) examination of the thyroid prior to a fine needle aspiration (FNA) has been described in patients with a clinically palpable nodule, since US may identify additional nodules in 20–48% of subjects (4).
Currently, ultrasound-guided FNA is the standard test to determine the course of action for a thyroid nodule. FNA is a relatively simple and noninvasive test; however, it should be used judiciously to limit its application for indeterminate and non-diagnostic nodules that may turn out to be benign. Therefore, the American Thyroid Association has recommended sonographic assessment of thyroid nodules before deciding whether the patient should undergo FNA (5). Additionally, the ultimate decision regarding operation in patients with a non-diagnostic FNA goes back to the initial clinician’s ultrasound assessment of the risk of malignancy.
High-resolution thyroid ultrasound has been widely used in the evaluation of thyroid nodules, resulting in the establishment of certain characteristics that differentiate benign and malignant thyroid nodules. US features predictive of a malignant nodule include intranodular vascularity, taller-than-wide shape, hypoechogenicity, irregular margins, and the presence of microcalcifications (6-10). However, some studies have found overlaps in the presence of these characteristics between benign and malignant nodules (11,12).
Furthermore, several studies have suggested that there is a significant association between malignancy and thyroid nodules demonstrating intranodular vascularity (7-9,13). The purpose of this study was to conduct a comparative analysis of Doppler ultrasound vascular flow between malignant and benign thyroid nodules using a systematic review and meta-analysis of current literature.
Methods
Identification of trials and data extraction
Articles published through March 2014 on PubMed, EMBASE, and the Cochrane Database of Systematic Review were systematically searched by two independent reviewers using the following medical subject headings (MeSH): “vascular thyroid nodule”, and “vascular malignant thyroid nodule”. Additionally, all articles included in the analysis underwent reference review for other potential articles. The inclusion criteria for eligibility were as follows: (I) articles comparing thyroid nodule vascularity in benign and malignant nodules; (II) studies that reported outcomes of vascular flow pattern, nodule size, calcifications, echogenicity, margins, and shape; (III) prospective studies, controlled clinical trials, or randomized controlled trials; (IV) studies that reported a measure of variance (standard error, standard deviation, or confidence interval); and (V) studies with a sample size of at least 50 nodules. Articles not reported in English were excluded. For research groups with redundant patient populations, the latest study on that population was included for analysis. Results from the two independent reviewers were compared for accuracy, with disagreement resolved by consensus.
Statistical analysis
Primary outcomes of this study included vascular flow pattern, nodule size, calcifications, echogenicity, margins, and shape. Odds ratios were calculated for categorical outcomes, while mean net changes were calculated for continuous variables. DerSimonian and Laird random-effects models were used to pool mean net changes or odds ratios across the studies (14). The presence of heterogeneity was assessed with Cochran’s Q test and its extent quantified with the I-square index. Funnel plots were constructed in order to assess publication bias and Begg’s rank correlation test was used to examine the asymmetry of these plots. Egger’s weighted linear regression test was used to examine the association between the mean effect estimate and its variance. Additionally, sensitivity analyses were conducted by excluding each study in turn, to evaluate their relative influence on the pooled estimates. All analyses were conducted in STATA (version 10; College Station, Texas, USA).
Results
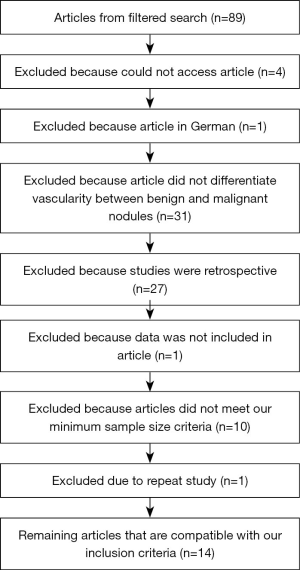
Searching the two databases, 89 publications were identified. Thirty-one were removed because the studies did not compare vascularity between benign and malignant nodules. Twenty-seven were removed because they were retrospective studies. Four were excluded because we were unable to access the articles. One was excluded because the article was in German. One study was excluded because the data was not included in the article. Ten were excluded because the sample size did not meet our required sample size. One study was excluded because it was a repeat study. The remaining 14 prospective studies underwent full text review and were included in final analysis (Figure 1).
In the 14 prospective studies that met inclusion criteria, a total of 4,154 thyroid nodules were evaluated. Of these, 1,419 (34%) were malignant. Thirty-three percent of these malignant thyroid nodules had no vascular flow, while 17% had peripheral flow and 50% had internal vascular flow (Table 1).
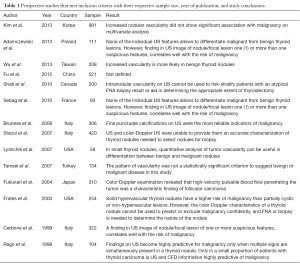
Full table
No vascular flow
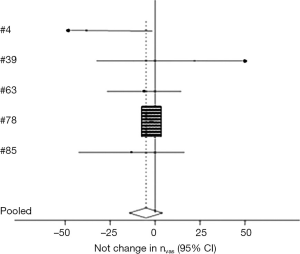
There were 12 studies (3,15-24) comprising 1,501 nodules that analyzed whether the absence of vascular flow correlated with thyroid malignancy with a range of 0% (22) to 56% (18). When meta-analysis was performed comparing benign and malignant nodules, five studies (9,15,16,22,24) were identified, which included 589 nodules. Analysis indicated that there was no significant difference in vascular flow between the two groups (95% CI: −14.329, 4.257) (Figure 2). The study by Kim et al. (15) was the only one demonstrating that lack of vascularity significantly indicated that nodules were benign.
Peripheral flow
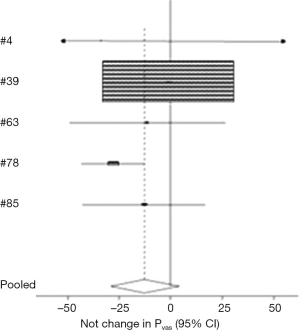
There were 11 studies (3,9,15-17,21-26) comprising 868 nodules that analyzed whether peripheral vascular flow correlated with thyroid malignancy with a range of 0% (22) to 69% (17). When meta-analysis was performed comparing benign and malignant nodules, five studies (9,15,16,22,24) were identified including 265 nodules. Analysis indicated that there was no significant difference in peripheral vascular flow (95% CI: −29.254, 4.313) between benign and malignant thyroid nodules (Figure 3). Frates (9) was the only study that found that peripheral vascularity significantly indicated that nodules were benign.
Intravascular
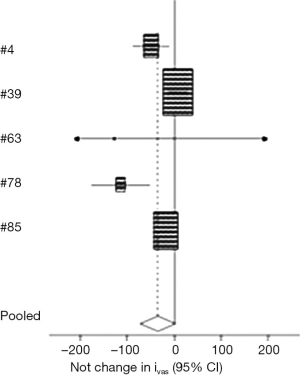
There were 14 studies (3,9,15-26) comprising 1,798 nodules that analyzed whether internal vascular flow correlated with thyroid malignancy with a range of 6% (22) to 76% (17). When meta-analysis was performed comparing benign and malignant nodules, five studies (9,15,18,22,24) were identified including 540 nodules. Analysis indicated that there was no significant difference in internal vascularity (95% CI: −72.067, 2.824) between malignant and benign thyroid nodules (Figure 4). Kim (15) and Frates (9) were the only studies that found that internal vascularity significantly indicated that nodules were benign.
Calcifications
There were seven studies (3,15,18-20,24,25) comprising 1,618 nodules that analyzed whether calcification had an effect on thyroid nodule malignancy. Only Shah (18) found that calcification had a significant effect on predicting malignancy on US.
Margins
Three studies (15,20,25) investigated the effect of margins on malignancy. There were 300 nodules found to have well-defined margins and 658 nodules with poorly-defined margins. Neither type of margin was found to have a significant effect on nodule malignancy.
Echogenicity
Six studies (3,15,19,20,24,25) investigated the effect of echogenicity on predicting thyroid malignancy. There were 1,230 nodules that were hypoechoic and 489 nodules that were hyperechoic. Echogenicity was not found to have a significant effect on nodule malignancy.
Consistency
Three studies (9,15,25) investigated the effect of nodule consistency on predicting thyroid malignancy. There were 894 solid nodules and 147 cystic nodules. Nodule consistency was not found to have a significant effect on malignancy.
Discussion
The high incidence of nodular thyroid disease and the low prevalence of malignancy require a diagnostic evaluation capable of screening the limited number of nodules that require surgery. Currently, a multidisciplinary approach based on clinical and laboratory data, ultrasonography, scintigraphy and FNA cytology are used, and 1 in 20 thyroid nodules operated on are found to be malignant (3). It has been our experience that increased nodule vascularity and ill-defined borers are associated with malignancy in indeterminate thyroid nodules. This meta-analysis of published articles suggests, however, that increased vascular flow on Doppler sonography does not accurately predict thyroid nodule malignancy. Other variables such as nodule size, calcifications, echogenicity, margins, and shape were also inaccurate for predicting thyroid nodule malignancy. Future studies are warranted to investigate the predictive role of such characteristics, including the degree of nodule vascularity, in diagnosing suspicious thyroid nodules as malignant or not.
Multiple studies (3,8,9,15-26) reviewed suggest that no individual ultrasound feature accurately differentiates benign and malignant nodules. Multiple suspicious features, however, do correlate with increased risk of malignancy. Therefore, increased vascularity can suggest increased risk of malignancy, especially when correlated with other suspicious findings, but is not itself diagnostic.
Wu et al. (16) suggested that objective quantified measures are required to diagnose malignancy; otherwise, the clinical implications of the ultrasound features are minor and non-diagnostic. Additionally, other studies have shown that malignant nodules are more likely to have internal vascularity while benign nodules are more likely to have peripheral vascularity (17,21,25). Further analysis is necessary to determine if specific patterns and types of internal or peripheral vascularity are associated with various thyroid malignancies.
Assessment of the other types of characteristics seen on US has been suggested by some investigators to be more reliable for predicting malignancy. Some studies have found that microcalcifications may be a more reliable predictor of malignancy (15,20,25). Brunese et al. (20) explained that this is due to psammoma bodies, which are around collections of calcifications commonly found in tumors such as papillary thyroid cancer. Others suggest that smaller nodules with vascularity are better predictors of malignancy (21). Further analysis of other suspicious features of malignancy alone and in conjunction with one another is necessary to determine if they may be more accurate predictors of malignancy.
Limited publications paired with the varying sample sizes of these studies necessitate further documentation of these outcomes before comparative inferences can be drawn on the accuracy of predicting thyroid malignancy by evaluating vascularity on Doppler sonography.
The results of this meta-analysis suggest that increased nodule vascularity does not accurately predict thyroid nodule malignancy. Therefore, ultrasound features alone cannot be used to avoid surgical intervention.
Acknowledgements
This work was fully supported by Tulane University Medical Center.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA 2006;295:2164-7. [Crossref] [PubMed]
- National Cancer Institute. SEER Stat Fact Sheets: Thyroid Cancer. Available online: http://seer.cancer.gov/statfacts/html/thyro.html. Accessed April 7, 2014
- Cerbone G, Spiezia S, Colao A, et al. Power Doppler improves the diagnostic accuracy of color Doppler ultrasonography in cold thyroid nodules: follow-up results. Horm Res 1999;52:19-24. [PubMed]
- Marqusee E, Benson CB, Frates MC, et al. Usefulness of ultrasonography in the management of nodular thyroid disease. Ann Intern Med 2000;133:696-700. [Crossref] [PubMed]
- American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167-214. [Crossref] [PubMed]
- Papini E, Guglielmi R, Bianchini A, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87:1941-6. [Crossref] [PubMed]
- Kim EK, Park CS, Chung WY, et al. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol 2002;178:687-91. [Crossref] [PubMed]
- Peccin S, de Castsro JA, Furlanetto TW, et al. Ultrasonography: is it useful in the diagnosis of cancer in thyroid nodules? J Endocrinol Invest 2002;25:39-43. [Crossref] [PubMed]
- Frates MC, Benson CB, Doubilet PM, et al. Can color Doppler sonography aid in the prediction of malignancy of thyroid nodules? J Ultrasound Med 2003;22:127-31. [PubMed]
- Moon WJ, Jung SL, Lee JH, et al. Benign and malignant thyroid nodules: US differentiation--multicenter retrospective study. Radiology 2008;247:762-70. [Crossref] [PubMed]
- Iannuccilli JD, Cronan JJ, Monchik JM. Risk for malignancy of thyroid nodules as assessed by sonographic criteria: the need for biopsy. J Ultrasound Med 2004;23:1455-64. [PubMed]
- Wienke JR, Chong WK, Fielding JR, et al. Sonographic features of benign thyroid nodules: interobserver reliability and overlap with malignancy. J Ultrasound Med 2003;22:1027-31. [PubMed]
- Kwak JY, Han KH, Yoon JH, et al. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology 2011;260:892-9. [Crossref] [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- Kim HG, Moon HJ, Kwak JY, et al. Diagnostic accuracy of the ultrasonographic features for subcentimeter thyroid nodules suggested by the revised American Thyroid Association guidelines. Thyroid 2013;23:1583-9. [Crossref] [PubMed]
- Wu MH, Chen CN, Chen KY, et al. Quantitative analysis of dynamic power Doppler sonograms for patients with thyroid nodules. Ultrasound Med Biol 2013;39:1543-51. [Crossref] [PubMed]
- Fu X, Guo L, Zhang H, et al. "Focal thyroid inferno" on color Doppler ultrasonography: a specific feature of focal Hashimoto's thyroiditis. Eur J Radiol 2012;81:3319-25. [Crossref] [PubMed]
- Shah MD, Conrad A, Ahmed A, et al. Decision making for the extent of thyroidectomy in the patient with atypical cytologic results. Arch Otolaryngol Head Neck Surg 2010;136:1177-80. [Crossref] [PubMed]
- Sebag F, Vaillant-Lombard J, Berbis J, et al. Shear wave elastography: a new ultrasound imaging mode for the differential diagnosis of benign and malignant thyroid nodules. J Clin Endocrinol Metab 2010;95:5281-8. [Crossref] [PubMed]
- Brunese L, Romeo A, Iorio S, et al. Thyroid B-flow twinkling sign: a new feature of papillary cancer. Eur J Endocrinol 2008;159:447-51. [Crossref] [PubMed]
- Lyshchik A, Moses R, Barnes SL, et al. Quantitative analysis of tumor vascularity in benign and malignant solid thyroid nodules. J Ultrasound Med 2007;26:837-46. [PubMed]
- Tamsel S, Demirpolat G, Erdogan M, et al. Power Doppler US patterns of vascularity and spectral Doppler US parameters in predicting malignancy in thyroid nodules. Clin Radiol 2007;62:245-51. [Crossref] [PubMed]
- Fukunari N, Nagahama M, Sugino K, et al. Clinical evaluation of color Doppler imaging for the differential diagnosis of thyroid follicular lesions. World J Surg 2004;28:1261-5. [Crossref] [PubMed]
- Rago T, Vitti P, Chiovato L, et al. Role of conventional ultrasonography and color flow-doppler sonography in predicting malignancy in 'cold' thyroid nodules. Eur J Endocrinol 1998;138:41-6. [Crossref] [PubMed]
- Adamczewski Z, Lewiński A. Proposed algorithm for management of patients with thyroid nodules/focal lesions, based on ultrasound (US) and fine-needle aspiration biopsy (FNAB); our own experience. Thyroid Res 2013;6:6. [Crossref] [PubMed]
- Stacul F, Bertolotto M, De Gobbis F, et al. US, colour-Doppler US and fine-needle aspiration biopsy in the diagnosis of thyroid nodules. Radiol Med 2007;112:751-62. [Crossref] [PubMed]