Robotic retroauricular thyroid surgery
Introduction
Thyroid nodules are common and found in up to 50–60% of the population (1). The chance of being diagnosed with thyroid cancer has risen in recent years, and it is the most rapidly increasing cancer in the US (2). Surgery is the gold standard treatment for patients with thyroid cancer or nodules suspicious for cancer.
Conventional open cervical approach is the standard surgical approach. However, concerns of a visible neck incision were raised, especially in young females or patients with history of healing with keloid or hypertrophic scar. As a consequence, several remote access endoscopic approaches have been described, including anterior chest (3), breast (4), transaxillary (5,6), bilateral axillo-breast approach (7), and postauricular-transaxillary approach (8). Afterwards, the robotic gasless-transaxillary approach was introduced in South Korea (9). This technique did overcome most of the endoscopic approach limitations, with better three-dimensional visualization and improved dexterity using endowristed instruments (10,11).
In 2011, Terris et al. introduced the robotic retroauricular approach for thyroid surgery using a facelift incision that is usually utilized in parotid surgeries. This approach is more familiar to head and neck surgeons who are not comfortable with tansaxillary approach (12,13).
This review will focus on the performance and safety of robotic retroauricular thyroid surgery.
Patient selection
Proper patients’ selection is the most important aspect in the robotic retroauricular approach, to assure a safe surgery without unnecessary harm. This depends on certain patients’ and disease characteristics. Non-obese patients (BMI <30) with concerns of a visible neck incision, especially patients with history of healing with keloid or hypertrophic scar are the best candidates. However, this approach can be also offered safely to obese patients. Previous neck surgery or radiations are considered contraindications to this remote access technique.
Regarding disease characteristics, this approach is only sufficient to perform thyroid lobectomy. Thyroid nodule should not be more than 4 cm, with no associated substernal or retropharyngeal goiter. Graves’ disease is an absolute contraindication, since these patients require total thyroidectomy. In cases of thyroid cancer, this approach should be offered only to patients with differentiated thyroid cancer, with no evidence of extrathyroid extension or pathological lymphadenopathy (14,15).
Surgical technique
Positioning and flap creation
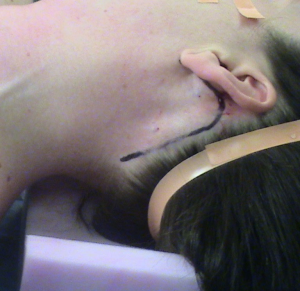
The patient is positioned supine with slight rotation of the head to the contralateral side of the planned incision, and both arms are tucked on the sides. Neck extension is not required in this approach. Intubation is performed using electromyography (EMG) endotracheal tube to allow intraoperative monitoring of the recurrent laryngeal nerve (RLN). Then small area of hair is shaved along the extension of the incision into the occipital hairline. Then the retroauricular incision is marked just posterior to the earlobe, extending into the postauricular crease, and adjacent to the occipital hairline at a position that will be obscured by the ear and hair (Figure 1). The flap is created deep to the platysma as described by Terris et al. (12,14). However, in our practice the flap is elevated superficial to the platysma with a special Metzenbaum scissor, which is an approach utilized by plastic surgeons in facelift procedures (15,16). In the condition of using this approach, surgeons have to be aware to avoid performing it on smoker patients. The flap dissection is continued until reaching the anterior border of sternocleidomastoid muscle (SCM). During dissection, the greater auricular nerve (GAN) and external jugular vein are identified and preserved. Then the plane between the strap muscles and anterior border of SCM is dissected and the SCM is retracted posteriorly, using army-navy retractor attached to a Greenberg retractor and secured to the ipsilateral side of the table. We have also described a modified approach, by creating the plane between the two heads of SCM, which eliminate the need of self-retaining retractor for SCM posterior retraction, similar to the transaxillary approach (15,16). This is followed by retraction of the omohyoid muscle ventrally and dissection of the strap muscles from the thyroid gland until reaching the contralateral thyroid lobe. A specially designated modified thyroidectomy retractor (Marina Medical, Sunrise, FL, USA) is then secured to the contralateral side of the table mount, and its blade is placed under the flap and strap muscles. This will maintain continuous exposure of the surgical field. The robot, Da Vinci Si or Xi system (Intuitive Surgical, Inc., Sunnyvale, CA, USA), is docked from the contralateral side, using 30-degree dual channel down viewing scope in the center, Maryland dissector in the nondominant hand, and a harmonic curved shears in the dominant hand.
Thyroid lobectomy
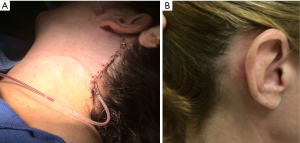
The upper thyroid pole is retracted ventrally and inferiorly and the superior thyroid vessels are then dissected and divided near the thyroid capsule using the Harmonic. Then the gland is retracted medially, and the RLN is identified in the tracheoesophageal groove. Its functional integrity is confirmed using nerve monitor stimulator. Then the nerve is dissected along its path until the insertion into the cricothyroid muscle, and a safe distance is created between it and the thyroid. Superior and inferior parathyroid glands are identified and preserved. The inferior thyroid pedicle is then dissected and divided distally using the harmonic. Subsequently the thyroid is dissected from the trachea, then the isthmus is divided and the thyroid removed through the retroauricular incision. We routinely place a drain; protruding posterior to the retroauricular incision. However, Dr. Terris described not using a drain in his experience (12). Interrupted subdermal closure is performed with 3–0 Vicryl suture. The skin at the hairline is closed with interrupted 5–0 Prolene sutures and staples (Figure 2). In many patients, we were able to offer additional excision of redundant skin which can provide a face lift in older patients in addition to thyroid surgery. Postoperatively patients are usually discharged on the same day, unless admission was indicated for other reason. However, this varies among surgeons depending on their usual practice.
Safety of the procedure
The risks associated with the retroauricular approach are similar to the open approach, such as, infection, hematoma, seroma, RLN injury and hypoparathyroidism. Several studies have reported the safety of the retroauricular approach (12,15-18), and showed comparable complication rate to the conventional open approach (19). On the other hand, there are other risks specific to this approach, which is injury to the flap or GAN. The surgeons have to be aware of those risks during the procedure to prevent them, by careful dissection of the flap and identified and preservation of the nerve. However, it was reported that some patients develop temporary hypoesthesia at the surgery site or the area corresponding to the distribution of GAN, in spite of preserving the nerve, which improve spontaneously (12,15).
Pros and cons
As most of thyroid surgeries are performed in young women, they have an understandable concern of the cosmetic outcome. This concern is more pronounced in patients with history of healing with keloid or hypertrophic scar formation. The retroauricular approach provides the patient with a hidden scar and a superior cosmetic satisfaction using a safe and feasible technique (12,15,18,19).
The use of the robot provides several advantages. The three-dimensional and magnified picture; makes the identification and dissection around the RLN and parathyroid gland easier (19). The wristed instruments and tremor filtration feature facilitate working in a small limited space with precise, minute movements (10,15). It also provides ergonomically perfect position for the surgeon (11,15,19,20). The longer operative time was the only disadvantage of this approach, and this is attributed to the time required for flap creation and robot docking (12,19). However, this is expected to improve with experience and improvement in robotic instruments (15,19).
In our experience, there is no difference in complication rates between retroauricular approach and the other most commonly used remote access robotic thyroidectomy approach; transaxillary. Some authors reported shorter operative time with retroauricular approach (17,19), as the distance between the incision and thyroid is shorter. Singer et al. reported that the surgical dissection area is less by 38% in the retroauricular approach compared to the transaxillary (13). However, these are operator dependent and are very small series. The positioning is easier which would save time but that is not significant in our experience. There is no concern of paresthesia of the anterior chest (17,19). The only limitation of retroauricular approach is that bilateral incision is required to perform total thryoidectomy, compared to one side approach in the transaxillary approach, which provides better exposure for bilateral surgery and central compartments.
Conclusions
In conclusion, retroauricular approach is a safe and feasible approach, and has a comparable outcome to the conventional open approach. In addition, it provides superior cosmetic satisfaction especially in young females with history of healing with keloid or hypertrophic scar. Advances in robotic instruments would help expand the selection criteria for surgery and possibly reduce the operative time.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gharib H, Papini E, Garber JR, et al. American association of clinical endocrinologists, American college of endocrinology, and associazione medici endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules--2016 update. Endocr Pract 2016;22:622-39. [Crossref] [PubMed]
- American Cancer Society. Available online: http://www.cancer.org/cancer/thyroidcancer/detailedguide/thyroid-cancer-key-statistics
- Ikeda Y, Takami H, Tajima G, et al. Total endoscopic thyroidectomy: axillary or anterior chest approach. Biomed Pharmacother 2002;56 Suppl 1:72s-78s. [Crossref] [PubMed]
- Ohgami M, Ishii S, Arisawa Y, et al. Scarless endoscopic thyroidectomy: breast approach for better cosmesis. Surg Laparosc Endosc Percutan Tech 2000;10:1-4. [Crossref] [PubMed]
- Ikeda Y, Takami H, Sasaki Y, et al. Endoscopic neck surgery by the axillary approach. J Am Coll Surg 2000;191:336-40. [Crossref] [PubMed]
- Kang SW, Jeong JJ, Yun JS, et al. Gasless endoscopic thyroidectomy using trans-axillary approach; surgical outcome of 581 patients. Endocr J 2009;56:361-9. [Crossref] [PubMed]
- Choe JH, Kim SW, Chung KW, et al. Endoscopic thyroidectomy using a new bilateral axillo-breast approach. World J Surg 2007;31:601-6. [Crossref] [PubMed]
- Lee KE, Kim HY, Park WS, et al. Postauricular and axillary approach endoscopic neck surgery: a new technique. World J Surg 2009;33:767-72. [Crossref] [PubMed]
- Kang SW, Jeong JJ, Yun JS, et al. Robot-assisted endoscopic surgery for thyroid cancer: experience with the first 100 patients. Surg Endosc 2009;23:2399-406. [Crossref] [PubMed]
- Lee J, Chung WY. Robotic thyroidectomy and neck dissection: past, present, and future. Cancer J 2013;19:151-61. [Crossref] [PubMed]
- Kang SW, Lee SC, Lee SH, et al. Robotic thyroid surgery using a gasless, transaxillary approach and the da Vinci S system: the operative outcomes of 338 consecutive patients. Surgery 2009;146:1048-55. [Crossref] [PubMed]
- Terris DJ, Singer MC, Seybt MW. Robotic facelift thyroidectomy: II. Clinical feasibility and safety. Laryngoscope 2011;121:1636-41. [Crossref] [PubMed]
- Singer MC, Seybt MW, Terris DJ. Robotic facelift thyroidectomy: I. Preclinical simulation and morphometric assessment. Laryngoscope 2011;121:1631-5. [Crossref] [PubMed]
- Terris DJ, Singer MC, Seybt MW. Robotic facelift thyroidectomy: patient selection and technical considerations. Surg Laparosc Endosc Percutan Tech 2011;21:237-42. [Crossref] [PubMed]
- Kandil E, Saeed A, Mohamed SE, et al. Modified robotic-assisted thyroidectomy: an initial experience with the retroauricular approach. Laryngoscope 2015;125:767-71. [Crossref] [PubMed]
- Saeed A, Alsaleh N, Moulthrop T, et al. Modified Approach for Robotic Retroauricular Thyroidectomy: Preclinical Simulation and a Surgical Case. Surg Innov 2015;22:577-81. [Crossref] [PubMed]
- Terris DJ, Singer MC. Qualitative and quantitative differences between 2 robotic thyroidectomy techniques. Otolaryngol Head Neck Surg 2012;147:20-5. [Crossref] [PubMed]
- Byeon HK. Comprehensive application of robotic retroauricular thyroidectomy: The evolution of robotic thyroidectomy. Laryngoscope 2016;126:1952-7. [Crossref] [PubMed]
- Lee DY, Lee KJ, Han WG, et al. Comparison of transaxillary approach, retroauricular approach, and conventional open hemithyroidectomy: A prospective study at single institution. Surgery 2016;159:524-31. [Crossref] [PubMed]
- Lee J, Kang SW, Jung JJ, et al. Multicenter study of robotic thyroidectomy: short-term postoperative outcomes and surgeon ergonomic considerations. Ann Surg Oncol 2011;18:2538-47. [Crossref] [PubMed]