Radioguided sentinel lymph node biopsy in patients with papillary thyroid carcinoma
Introduction
The American Thyroid Association (ATA) guidelines do not recommend prophylactic central compartment neck dissection in patients with T1 and T2 papillary thyroid carcinoma (PTC) with no clinical evidence of lymph node metastasis, but recommend AJCC/TNM staging for all PTC patients (1). Unfortunately, preoperative ultrasound identifies suspicious cervical lymphadenopathy in 20–30% of patients with PTC (2,3), and the lymph node staging is required for the AJCC/TNM classification (4) thus, following the ATA guidelines, patients with small tumor will be classified as pNx because the regional lymph nodes cannot be assessed histologically.
Sentinel lymph node biopsy (SLNB) is widely accepted as the standard of care in patients with breast cancer (5) and clinically localized malignant melanoma (6) for correct lymphatic basin staging and for selection of patients who can benefit from lymph node dissection (LND). However, the role of SLNB in the treatment of PTC remain open to debate. Rajmakers et al. in a meta-analysis on sentinel lymph node (SLN) detection in patients with thyroid cancer, with the aim to determine the technique (dye versus radioguided surgery) that demonstrated the highest success rate, indicated that studies using the radioguided technique yielded an approximately 13% higher SLN detection rate compared with those using the dye technique (7), and this data are reinforced by a most recent meta-analysis (8). Furthermore, the injection of the radiotracer for radioguided surgery is performed preoperatively, thus this strategy eliminates the potential disruption of lymphatic channel during the initial dissection that normally occurs with SLNB using the intraoperative injection of dye. This findings support the use of radioguided technique instead of dye method in thyroid cancer patients. Some Authors have proposed, like in breast cancer and melanoma, a radioguided SLNB (rSLNB) (9) with SLN frozen section to identify patients with SLN metastasis in whom LND of the neck compartments should be performed and those with negative SLN in whom the LND may be avoided. By contrast, other Authors (10) suggested a new concept of radioguided surgery for lymph node staging of PTC patients, based on the use of SLNB only as a guide to perform a selective LND of the SLN compartment (i.e., central or lateral neck compartments) regardless of the SLN status, due to the high false negative rate (FNR) of the rSLNB carried out alone.
The aim of this systematic review was to evaluate the role of rSLNB in the treatment of PTC patients, with a particular interest on the safety of the procedure and the impact on lymph node staging.
Methods
Eligibility criteria
This systematic review was performed following the Preferred Reporting Items for Systematic review and Meta-Analysis Protocols (PRISMA-P) 2015 statement (11). Using the PICO (Participants, Intervention, Comparison, Outcomes and design) method, the criteria for selection of the studies were:
- Participants: studies of patients with PTC;
- Intervention: studies in which PTC patients were treated by rSLNB performed by a radiotracer (e.g. 99mTechnetium; 99mTc);
- Comparator: a control group was not necessary due to the primary and secondary outcomes;
- Outcomes: the primary outcome was the FNR of the rSLNB to evaluate the safety of the procedure. The secondary outcomes were the intraoperative identification rate (IIR) of the SLN, the site of both SLN and lymph node metastasis within the neck compartments to define the possible role of rSLNB for lymph node staging; and the persistent disease during follow up to determine the safety of the rSLNB associated to LND in the same compartment of the SLN;
- Type of study: randomized control trial (RCT), retrospective and prospective cohort study, retrospective and prospective observational study, regardless of the number of patients involved. In case of studies in which PTC patients treated by rSLNB were compared to other techniques, only patients who underwent rSLNB were included in the review analysis, due to the aims of this systematic review.
No limitation regarding publication date was considered; only articles in English language were included.
The exclusion criteria were: (I) SLNB performed by a dye technique or other methods; (II) studies without original data (i.e., letter to the editor, review, editorial); (III) abstract accepted for presentation at a national/international meeting due to the insufficient patients’ data available in the abstract.
Among the studies included in the review, we excluded from the analysis: (I) patients without PTC; (II) patients who underwent SLNB performed by a dye technique alone, used in the study as a control group; (III) patients who underwent a central compartment LND without rSLNB, used in the study as a control group.
Information sources and search strategy
A systematic search was performed on July 17, 2016 in the PubMed database and Embase database to identify all original articles regarding the application of rSLNB in PTC patients. The searched terms were:
- ‘sentinel lymph node’ OR ‘radioguided surgery’ OR ‘radioguided’ OR ‘radio-guided’ OR ‘gamma-probe’ AND ‘papillary thyroid cancer’;
- ‘sentinel lymph node’ OR ‘radioguided surgery’ OR ‘radioguided’ OR ‘radio-guided’ OR ‘gamma-probe’ AND ‘papillary thyroid carcinoma’.
Study records
Titles and abstracts of all identified articles were screened by two independent investigators (MP, PC), and the articles meeting the inclusion criteria were deeply analyzed in the full-text version. Multiple articles from the same authors and institutions were evaluated carefully for possible duplication, and if this was happened, only the most recent article was included to avoid duplication of patients’ data.
Data were recorded according to the PICO method and, based on the outcomes of the present review, we have reported the FNR of the rSLNB, the IIR of the SLN, the site of both SLN and lymph node metastasis within the neck compartments, and the persistent disease (i.e., lymph node metastases within the first 12 months of follow-up). During the analysis of the selected studies, if the FNR was erroneously considered by the Authors as the rate of false negative cases over the entire group of patients, the FNR was calculated with a rigorous formula as previously reported (10,12). Briefly, the FNR for rSLNB was calculated as the number of patients with negative SLN but positive non-SLN after LND in the same compartment of the SLN (i.e., false negative cases) over the sum of the true positive (i.e., patients with positive SLN) plus the false negative cases, multiplied by 100. The FNR for the radioguided LND in the SLN compartment (i.e., radioguided selective compartment neck dissection; RSCND) was calculated as the number of patients with both negative SLN and non-SLN after RSCND who developed lymph node metastases within the first 12 months of follow-up (i.e., false negative cases) over the sum of the true positive (i.e., patients with either positive SLN or NSLN or both) plus the false negative cases, multiplied by 100. The FNR of SLN frozen section was considered as the number of patients with negative SLN at intraoperative pathological examination but positive SLN at final pathology (i.e., false negative cases) over the sum of the true positive (i.e., patients with positive SLN at frozen section) plus the false negative cases, multiplied by 100.
Risk of bias in individual studies
A control group was not necessary due to the primary and secondary outcomes of this review and only PTC patients who underwent rSLNB were included in the review analysis without a control group, thus the evaluation of the bias risk with the Newcastle-Ottawa scale (13) for the case-control or cohort studies and the Jadad scale (14) for RCT was not applicable.
Statistical analysis
Data are presented as a median (range) or mean (standard deviation). Descriptive analyses were performed by using IBM SPSS Statistics for Windows, Version 24.0 (IBM Corp. Armonk, NY: IBM Corp).
Results
Selection process of the articles
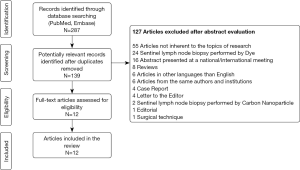
After duplicates removal, 139 potentially relevant records were identified and 127 of these articles were excluded (Figure 1). The aims of the 12 articles included in the review analysis were: (I) to evaluate the feasibility of rSLNB in four studies (15-18); (II) to compare the identification of SLN with rSLNB versus SLNB performed by a dye technique in two studies (19,20); and (III) to determine the role of rSLNB for PTC lymph node staging in six studies (9,10,21-24). Only PTC patients who underwent rSLNB were included in the analysis, achieving a total of 800 patients. Among the 12 studies included, only one involves >300 patients, two ≥100 patients, and all the others comprise <50 patients. Despite the low number of patients, three case series were included in the review as the first historical experiences on rSLNB.
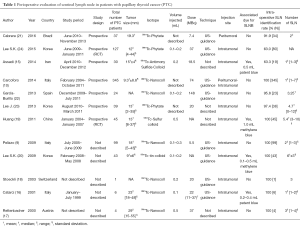
The vast majority of PTC patients of the included studies had a tumor size ≤40 mm (T1–T2) (Table 1).
Full table
Radioguided sentinel lymph node biopsy
Injection site and intraoperative identification rate of SLN
An intratumoral injection of the radiotracer was used in 10 studies (9,15-20,22-24) with a median IIR of 100% (63–100%); in one study it was injected in the peritumoral space with an IIR of 91.9% (21); in one study the radiotracer was injected peritumorally in the first study period and then intratumorally to improve the preoperative visualization with an IIR of 100% (10) (Table 1). The IIRs of the included studies are shown in Table 1. Overall, the SLN was identified in 737 out of 800 patients and the overall SLN IIR with the radioguided technique was 92.1%.
Site of SLN within the neck compartments
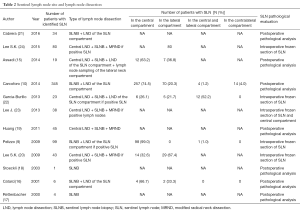
Table 2 shows the distribution of the SLN within the neck compartments in the different studies included in the review. Not all studies have described the SLN basin in the neck, and in 122 out of 737 patients (16.5%) it was not available (17-19,21,23). Among all other patients the SLN was in the central compartment in 53.1% (391/737), in the lateral one in 26.2% (193/737), in both central and lateral compartments in 2.3% (17/737), and in the contralateral compartments in 1.9% (14/737).
Full table
Type of LND associated to SLNB
As shown in Table 2, a LND in the same compartment of the SLN regardless of the SLN status was performed in 5 out of 12 studies (449 patients; 60.9%) (10,15,16,19,21). Instead, in five studies the LND of the SLN compartment was done if positive SLN at intraoperative frozen section (283 patients; 38.4%) (9,20,22-24). In two case series only the SLN was removed (5 patients; 0.7%) (17,18).
Lymph node metastasis
SLN pathological evaluation
Among five studies in which a frozen section of the SLN was performed, the FNR of intraoperative pathological examination were 17.2% (24), 8.3% (22), and 9.5% (20). In two studies the false negative cases of SLN frozen section were not available (9,23). In all other studies a postoperative pathological evaluation was done (Table 2).
SLN status and lymph node metastasis within the neck compartments
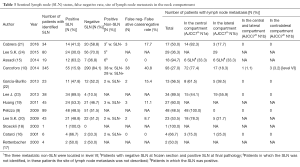
The SLN status and site of lymph node metastasis within the neck compartments of the included studies are summarized in Table 3. The overall SLN metastatic rate was 33.6% (248/737); the overall lymph node metastatic rate was 38.6% (309/800). In 36 out of 309 patients (4.5%), the site of lymph node metastasis was not available, and then the lymph node metastatic rates within the neck compartments were 23.0% in the central compartment (184/800); 10.6% in the lateral compartments (85/800); 0.1% in both central and lateral compartments (1/800); and 0.4% in the contralateral compartments (3/800).
Full table
False negative rate
Table 3 shows the FNR of the included studies in which a LND in the same compartment of the SLN was performed and the false negative cases were available, thus the overall FNR was 25.4% (48/189).
Persistent and recurrent disease
Follow up was available only in three studies; the persistent and recurrent disease are shown in Table 4.
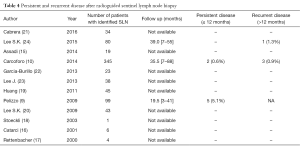
Full table
Discussion
This systematic review shows that in PTC patients, also in T1–T2 stage, the rSLNB is a feasible technique with an high intraoperative identification rate that allow to identify the possible lymphatic pathway of tumor cells within the neck compartments (i.e., central or lateral). In fact, lymph node metastasis prevail in the central compartment, but the lateral and contralateral compartments may be involved. However, the rSLNB performed alone is burdened by a high FNR that may lead a lymph node understaging with a possible increased risk of persistent disease during follow up. An intraoperative pathological evaluation of the SLN to avoid LND in patients with negative SLN is not recommended due to the high FNR of the frozen section.
Injection site and intraoperative identification rate of SLN
In two studies (15,24) in which the radiotracer was injected intratumorally, the IIR was low (63%) and this may be determined by two main issues: (I) an intratumoral injection theoretically could decrease the SLN detection rate due to poor lymphatic drainage from the tumor parenchyma compared to the peritumoral area; and (II) the learning curve effect may be an important parameter in the SLN mapping success. However, in the other 8 studies in which an intratumoral injection was performed the IIR was high (median 100%; range, 95.8–100%) (Table 1). Thus, in light of that, the learning curve effect seems more important than the poor intratumoral lymphatic drainage for the intraoperative radioguided identification of the SLN. Furthermore, Carcoforo et al. injected the radiotracer peritumorally in the first study period and then intratumorally to improve the preoperative visualization, but the IIR for PTC patients was 100% over the entire study period (10) (Table 1).
Therefore, an intratumoral injection of the radiotracer seems a feasible technique with a high intraoperative identification rate.
SLN status, lymph node metastasis within the neck compartments, and FNR of SLN frozen section
The vast majority of patients involved in the included studies had T1 and T2 PTC (Table 1), and based on the ATA guidelines (1) in these patients a LND would not be recommended, however the rSLNB allowed to identify occult lymph node metastasis in the central, lateral and contralateral neck compartments (Table 3), and then to improve lymph node staging. Generally, the central neck compartment is considered the preferential pathway of PTC lymph node metastasis, however the lateral neck compartments were involved by tumor cells in a not irrelevant percentage of PTC patients, and this was discovered through the rSLNB. Thus, following the ATA guidelines these PTC patients would have been staged as lymph node negative, and not submitted to radioiodine ablation. These findings are underlined in the studies performed by Cabrera (21), Lee SK (24), Huang (19), and Carcoforo (10) in which if the ATA guidelines had been followed by the Authors, respectively 70.6% (21), 100% (24), 100% (19) and 98.9% (10) of patients with lymph node metastasis would have not been submitted to LND due to the tumor stage T1–T2, and they would have been understaged; thus a persistent disease would have expected in these patients.
Due to the high FNR of the SLN frozen section, this procedure is not recommended to avoid LND during the surgical operation for papillary thyroid cancer.
FNR of rSLNB and LND
The FNR is the single most important quality item for the SLN technique, and it may influence the persistent disease during follow up. If a negative SLN biopsy could not definitively exclude the presence of positive basin lymph nodes, it adds no further staging information.
Thus, to evaluate the safety of the rSLNB in the treatment of PTC patients, a LND in the same compartment of the SLN should be performed regardless of the SLN status. In only 5 out of 12 studies a LND of the SLN compartment was performed and the overall FNR was high (25.4%).
Pelizzo et al. in 99 patients performed the LND of the same compartment of the SLN only if the SLN was positive at the frozen section, thus it is not possible to identify patients with negative SLN and metastasis in other lymph node of the same neck compartment for the evaluation of the false negative cases (9). However, the Authors suggested that it is necessary to remove not only the hottest SLN but all nodes with a count higher than 10% of the hottest node, because in their study population among 48 patients with SLN metastasis, in 32 patients the hottest SLN (1st SLN) was positive and in 16 patients were positive the 2nd and/or the 3rd SLN (less radioactive) and not the first one (9). This findings support the hypothesis that in the treatment of PTC the removal of one SLN, contrary to the breast cancer and melanoma, it is not safe due to the possible presence of metastases in other lymph node of the same compartment without involvement of the hottest SLN.
Huang et al. showed that a radioguided technique allow to acquire a 100% of SLN identification rate and a low, but not negligible, FNR removing a high mean number of SLN (19). By contrast, Carcoforo et al. in the largest study on rSLNB in PTC patients showed the same identification rate (100%) but, if the rSLNB is applied like in breast cancer or melanoma by removing the lowest number of SLN for the identification of the true first lymph node that drain the primary tumor (e.g., median of 1 SLN), the FNR increase exponentially to about 40%, showing that for the treatment of PTC, the rSLNB by itself may be abandoned unless the introduction of a new role of radioguided surgery (10). Due to the high FNR of rSLNB, the Authors introduced a new concept of the application of the radioguided technique in the treatment of PTC, proposing a radioguided selective compartment neck dissection (RSCND) performed only in the same compartment of the SLN without both a frozen section of the SLN and a routine/prophylactic central LND. The FNR of such a technique, based on the persistent disease during the first 12 months of follow up, was 1.1% suggesting the safety of the procedure. This new approach may safely increase the identification of patients with lymph node metastasis and possibly reduce the persistent disease, and may avoid a useless central LND in some patients due to the lymphatic drainage in the lateral compartment (10). Thus, the Authors concluded that according to all such data, also considering the high FNR of rSLNB, the radioguided technique may well be applied to PTC patients to guide the compartment oriented lymphadenectomy by RSCND, to increase the metastatic yield and improve staging of the disease, rather than to avoid a prophylactic lymphadenectomy by rSLNB based on intraoperative SLN frozen section. In addition, refining the staging by RSCND allows improvement of the selection of patients for postoperative radioiodine ablation which may potentially reduce the persistent disease rate after operative intervention (10).
This findings suggest that a negative rSLNB performed alone may not exclude the presence of positive basin lymph nodes, thus radioguided surgery may be used to identify the potential pathway of the metastatic cells within the neck compartments, and then to guide the compartment oriented lymphadenectomy.
Persistent and recurrent disease
Locoregional recurrence, especially the persistent disease, has been suggested as a valid endpoint to evaluate the effectiveness of therapy for PTC (25). A persistent disease within the first 12 months of follow up after surgical removal of the primary tumor may depend on a lack of dissection of metastatic lymph node during the first operation that will appear during follow up. This condition may occur in patients who underwent either a prophylactic central compartment LND or a rSLNB performed alone.
Recently, Viola et al. showed a persistent disease of 7.5% in patients who underwent a prophylactic central compartment LND (26), and this result is supported by Durante et al. in a previous multicenter study in which a persistent disease was 7.1% (27). Furthermore, Carcoforo et al. showed an high FNR of the rSLNB performed alone and they pointed out the risk of leaving lymph node metastasis in the neck, due to the negativity of the SLN, that may clinically appear during follow up as a persistent disease (10). Interestingly, Pelizzo et al. that performed the LND of the same compartment of the SLN only if the SLN was positive at the frozen section showed a persistent disease of 5.1%, and it may be influenced by the FNR of frozen section and the FNR of rSLNB performed alone (9). Instead, Carcoforo et al. carried out a compartment oriented LND guided by the SLN and showed a persistent disease of 0.6% (10). Thus, it might be safer to perform a compartment oriented LND in the same compartment of the SLN, regardless of the SLN status, and analyze all the removed lymph nodes at final histopathological evaluation for better staging of the disease and potentially reduce the persistent disease.
Pros and Cons of the rSLNB are summarized in Table 5.

Full table
Limitation of the systematic review
This systematic review has some limitations which have to be pointed out. Firstly, the vast majority of the included studies had a low number of patients involved and it may influence the IIR and the FNR of the rSLNB due to the learning curve effect. Secondly, not in all studies a LND of the SLN compartment was performed, and then it may condition the real overall FNR. Thirdly, nowadays there are no randomized control trials that compare the recent ATA guidelines (1) and the RSCND to evaluate the advantage of the latter technique in term of persistent and recurrent disease, and such a randomized control trial will be required to clarify the role of the radioguided surgery in the treatment of PTC patients. Fourthly, the available validated scales for quality assessment of the included studies in the systematic review were not applicable, thus bias risk may be expected. Fifthly, only articles in English language were included.
Conclusions
In all PTC patients, also in T1–T2 stage in which lymph node metastasis within the neck compartments may occur, due to the high FNR, the rSLNB performed alone should be abandoned and converted into a technique to guide the lymphadenectomy in a specific neck compartment (i.e., central or lateral), regardless of the SLN status and the tumor size. The radioguided selective compartment neck dissection (RSCND) seems a safe and promising technique which allow clinicians: (I) to identify PTC patients with lymph node metastasis within the neck compartment for better lymph node staging; (II) to improve a selective intraoperative tumor clearance; (III) to select patients for postoperative radioiodine ablation; and (IV) to potentially reduce the persistent disease rate.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Shimamoto K, Satake H, Sawaki A, et al. Preoperative staging of thyroid papillary carcinoma with ultrasonography. Eur J Radiol 1998;29:4-10. [Crossref] [PubMed]
- Solorzano CC, Carneiro DM, Ramirez M, et al. Surgeon-performed ultrasound in the management of thyroid malignancy. Am Surg 2004;70:576-80; discussion 580-2. [PubMed]
- Edge SB, Byrd SR, Compton CC, et al., editors. AJCC Cancer Staging Manual. 7th edition Springer-Verlag; New York (NY): 2010:143-64.
- Senkus E, Kyriakides S, Ohno S, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26 Suppl 5:v8-30. [Crossref] [PubMed]
- Mozzillo N, Caracò C, Chiofalo MG, et al. Sentinel lymph node biopsy in patients with cutaneous melanoma: outcome after 3-year follow-up. Eur J Surg Oncol 2004;30:440-3. [Crossref] [PubMed]
- Raijmakers PG, Paul MA, Lips P. Sentinel node detection in patients with thyroid carcinoma: a meta-analysis. World J Surg 2008;32:1961-7. [Crossref] [PubMed]
- Balasubramanian SP, Harrison BJ. Systematic review and meta-analysis of sentinel node biopsy in thyroid cancer. Br J Surg 2011;98:334-44. [Crossref] [PubMed]
- Pelizzo MR, Toniato A, Sorgato N, et al. 99Tc nanocolloid sentinel node procedure in papillary thyroid carcinoma: our mono-institutional experience on a large series of patients. Acta Otorhinolaryngol Ital 2009;29:321-5. [PubMed]
- Carcoforo P, Portinari M, Feggi L, et al. Radio-guided selective compartment neck dissection improves staging in papillary thyroid carcinoma: a prospective study on 345 patients with a 3-year follow-up. Surgery 2014;156:147-57. [Crossref] [PubMed]
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [Crossref] [PubMed]
- Nieweg OE. False-negative sentinel node biopsy. Ann Surg Oncol 2009;16:2089-91. [Crossref] [PubMed]
- Cota GF, de Sousa MR, Fereguetti TO, et al. Efficacy of anti-leishmania therapy in visceral leishmaniasis among HIV infected patients: a systematic review with indirect comparison. PLoS Negl Trop Dis 2013;7:e2195. [Crossref] [PubMed]
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [Crossref] [PubMed]
- Assadi M, Yarani M, Zakavi SR, et al. Sentinel node mapping in papillary thyroid carcinoma using combined radiotracer and blue dye methods. Endokrynol Pol 2014;65:281-6. [Crossref] [PubMed]
- Catarci M, Zaraca F, Angeloni R, et al. Preoperative lymphoscintigraphy and sentinel lymph node biopsy in papillary thyroid cancer. A pilot study. J Surg Oncol 2001;77:21-4; discussion 25. [Crossref] [PubMed]
- Rettenbacher L, Sungler P, Gmeiner D, et al. Detecting the sentinel lymph node in patients with differentiated thyroid carcinoma. Eur J Nucl Med 2000;27:1399-401. [Crossref] [PubMed]
- Stoeckli SJ, Pfaltz M, Steinert H, et al. Sentinel lymph node biopsy in thyroid tumors: a pilot study. Eur Arch Otorhinolaryngol 2003;260:364-8. [Crossref] [PubMed]
- Huang O, Wu W, Wang O, et al. Sentinel lymph node biopsy is unsuitable for routine practice in younger female patients with unilateral low-risk papillary thyroid carcinoma. BMC Cancer 2011;11:386. [Crossref] [PubMed]
- Lee SK, Choi JH, Lim HI, et al. Sentinel lymph node biopsy in papillary thyroid cancer: comparison study of blue dye method and combined radioisotope and blue dye method in papillary thyroid cancer. Eur J Surg Oncol 2009;35:974-9. [Crossref] [PubMed]
- Cabrera RN, Chone CT, Zantut-Wittmann DE, et al. The Role of SPECT/CT Lymphoscintigraphy and Radioguided Sentinel Lymph Node Biopsy in Managing Papillary Thyroid Cancer. JAMA Otolaryngol Head Neck Surg 2016;142:834-41. [Crossref] [PubMed]
- Garcia-Burillo A, Roca Bielsa I, Gonzalez O, et al. SPECT/CT sentinel lymph node identification in papillary thyroid cancer: lymphatic staging and surgical management improvement. Eur J Nucl Med Mol Imaging 2013;40:1645-55. [Crossref] [PubMed]
- Lee J, Na KY, Lee J, et al. The usefulness and accuracy of sentinel lymph node biopsy using single photon emission computed tomography/computed tomography with 99mTc phytate to detect locoregional lymph node metastases in patients with papillary thyroid carcinoma. J Korean Surg Soc 2013;84:195-201. [Crossref] [PubMed]
- Lee SK, Lee JH, Bae SY, et al. Lateral neck sentinel lymph node biopsy in papillary thyroid carcinoma, is it really necessary? A randomized, controlled study. Surgery 2015;157:518-25. [Crossref] [PubMed]
- Sakorafas GH, Sampanis D, Safioleas M. Cervical lymph node dissection in papillary thyroid cancer: current trends, persisting controversies, and unclarified uncertainties. Surg Oncol 2010;19:e57-70. [Crossref] [PubMed]
- Viola D, Materazzi G, Valerio L, et al. Prophylactic central compartment lymph node dissection in papillary thyroid carcinoma: clinical implications derived from the first prospective randomized controlled single institution study. J Clin Endocrinol Metab 2015;100:1316-24. [Crossref] [PubMed]
- Durante C, Montesano T, Torlontano M, et al. Papillary thyroid cancer: time course of recurrences during postsurgery surveillance. J Clin Endocrinol Metab 2013;98:636-42. [Crossref] [PubMed]