Small refinements in breast reconstruction: a technique for inframammary fold creation
Introduction
Breast cancer remains a major cause of cancer deaths among women worldwide. Advancements in treatments, including the broad application of oncoplastic and breast-conserving surgeries, have allowed stable and fairly high rates of patient survival with better quality of life. Mastectomies represent approximately 20–33% of all breast surgeries (1-3), and this cohort often requires breast reconstruction in order to save/restore their body image.
The aim of breast reconstruction is to achieve an aesthetically pleasant breast that matches, as much as possible; the idea of what a “normal breast” is (4). The definition of a “normal breast” was recently proposed by Blondeel et al. (5), who stated that breast identity can be defined by a balanced combination of four components: breast footprint, breast conus, breast skin envelope, and nipple-areola complex. The reconstruction of all of these components allows the natural shape of the breast to be recreated. The absence of even one element results in an unnatural-looking breast. One of the vital constituents of the breast footprint is the inframammary fold (IMF), which plays a significant role in forming and maintaining the shape of the breast. In cases in which the IMF is poorly defined, asymmetry with the contralateral breast or the disposition of all elements of the breast footprint can be observed, including changes in the contours of the upper and lower poles and their interference with the thoracic wall (6). Thus, in cases of absent IMFs, reconstruction becomes completely necessary during breast reconstruction, and as vital as nipple-areola complex reconstruction or contralateral breast symmetrization.
Unfortunately, the IMF is one of the most difficult anatomical structures to recreate during breast reconstruction surgery. This is particularly problematic because the IMF largely determines the final aesthetic results of breast reconstruction for the reasons explained above.
There are many proposed methods of IMF reconstruction (7-14) in the literature, but the majority of these techniques has some common disadvantages: visible scarring (7,8,10) and contour irregularities or indentations at the IMF (13), little ptosis improvement or small projection of reconstruction breast (6,8). The aim of this study was to report a new technique of IMF reconstruction that can provide stable results and improve the overall aesthetic results of breast reconstruction surgery.
Methods
Patients
Our original technique of IMF creation was used in 321 patients during two-stage breast reconstruction from January 2006 to January 2016 at the Breast Surgery Department of the Regional Clinical Cancer Center, Kazan, Russia. The median patient age was 38 years (range, 18–58 years). At our center, we mainly perform immediate reconstructions (87% of all reconstructions from 2000–2015 were immediate reconstructions) due to the availability of our team of plastic and cancer surgeons. Our center is considered to be a reference center for the Privolzhskiy Federal District of Russia, and we receive many referrals of patients from other regions of Russia for delayed reconstructions. As a result, we performed delayed reconstruction in 146 patients (45.5%) and immediate reconstruction in the remaining 175 (54.5%) patients. Indications for the application of our technique for IMF reconstruction were the absence of an IMF after mastectomy, two-staged breast reconstruction with an expander/implant, and signed informed consent by the patient. Patients with infection, abscess, seroma, or implant extrusion were not operated on until the specific problem had been resolved. In cases of wound complications or a lack of response to the provided treatment (antibiotics, corrective surgeries), patients were excluded from the study.
The study protocol was approved by the Ethics Committee of the Kazan State Medical Academy (ID: 5/12 from 03.12.2014) and conformed to the principals of the Declaration of Helsinki and its amendments. All patients provided written informed consent before participation on the study.
In the preoperative period, we performed standard evaluations including the assessment of general health status, electrocardiography, ultrasonographic imaging of the contralateral breasts, implants, and regional lymph nodes, blood analysis and urinalysis, and chest radiography with further consultation with internists and anesthesiologists.
Procedures
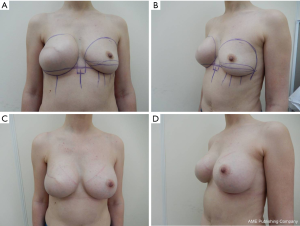
Before surgery, pre-operative markings were made with the patient standing (Figure 1A-D).
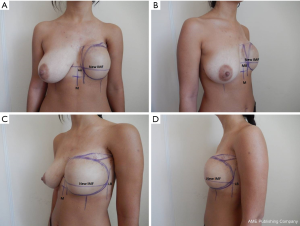
The midline, lateral, and medial borders (MBs) of the expander pocket were defined and marked. The location of the new IMF was marked on the horizontal line passing across the IMF of the contralateral breast. The expander/implant replacement stage of breast reconstruction was performed under general anesthesia. After removing the previously marked mastectomy scar tissue and extracting the expander, the anterior sheet of the capsule was removed, except for the lowest 2-cm. Two needles were placed: one on the MB at the intersection point of the new IMF with the MB of the expander pocket, and one at the IMF intersection with the LB (Figure 2A). Within the pocket, horizontal dissection of the posterior sheet (PS) of the capsule was performed under direct visual control from one needle to the other (1–2 cm above the lower transition fold of the expander pocket), after which the sheet was mobilized downwards (Figure 2B).
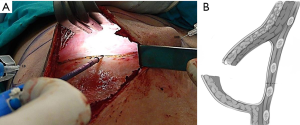
The PS of the capsule was then dissected downwards to the costal margin (Figure 3).
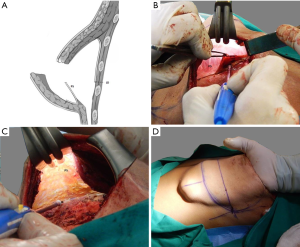
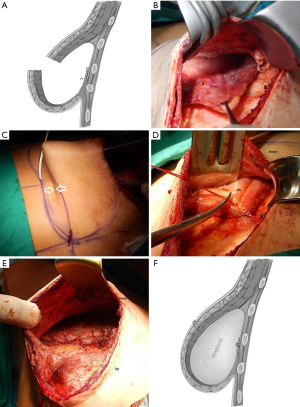
After dissecting the PS of the capsule, we pulled it upwards to free it (Figure 4A,B) until we could visually detect the crease just on the line where the new IMF should be placed (Figure 4C). At this point, we fixed this free border to the underlying tissues with non-absorbable interrupted sutures (Figure 4D,E,F). Before permanent implant placement, we preferred to check the definition of the IMF fold with the aid of the patients’ expander, which was treated with an antiseptic after extraction. The patient was maintained in a semi-sitting position to verify the correct position of the new IMF. Then, we returned the patient to the supine position, and after removing the expander for the final time, we placed the permanent textured implant and set one drain into the pocket. The layers over the implant were closed in the conventional manner.
Results
Although an IMF was not present before expander replacement (Figure 5A,B), we were able to produce natural looking breasts with well-defined IMFs accompanied with sharp (<90°) breast-thoracic angles and adequate symmetry with the contralateral breast (Figure 5C,D).
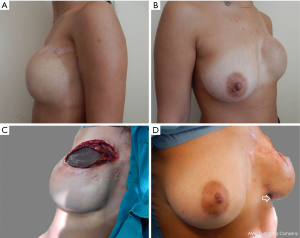
The mean duration of surgery was 95 min (range, 80–120 min). The patients were followed up for 5–6 years: every 3 months in the first postoperative year, then once every 6 months during the subsequent 3 years, and finally annually for 2 years, after which they were free to refer to us only in the case of complaints.
No serious complications in terms of infection, necrosis, or other issues associated with the method of reconstruction were observed.
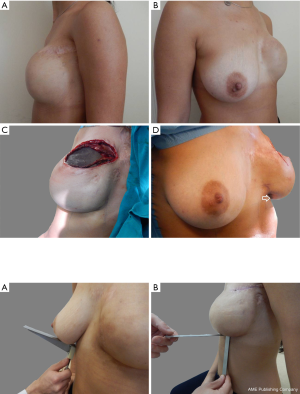
In terms of aesthetic results, we assessed the stability of the results by measuring breast-thoracic angle (Figure 6A) and the distance between the IMF and the lower border of segment III of the breast (Figure 6B). Measurements were carried out with minimal pressure applied to the skin.
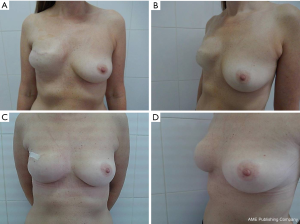
We also assessed the results by photographing the patients during check-ups in five standard positions from the same distance (Figures 7,8).
In two patients (0.62%) who were operated on early in our experience of using this method, we noticed unstable results with partial distortion of the IMF 4 months after surgery, which we eventually associated with the use of absorbable sutures. After amending the procedure to use only non-absorbable sutures, we did not notice any more instances of this complication. Another complication was implant rotation, which was also uncommon [five patients (1.6%) from 2006–2008)] and was associated with the pocket, which we intentionally made slightly larger than the implant. With the use of polyurethane-covered implants, we have managed to prevent any more occurrences of this complication.
We noticed that breast-thoracic angle decreased in only 21 patients (6.5%). In 14 patients (4.4%), we noticed an increase in the distance from the IMF to the lower border when we compared the reconstructed breast to the contralateral breast. We observed this complication during check-ups in the first year after surgery, and after 1 year, this distance stabilized. Both complications were associated with the minor ptosis of the reconstructed breast with regard to IMF. We did not notice any cases of an increase in breast-thoracic angle or the absence of natural-looking ptosis at the 1-year follow-up.
Discussion
The first attempts to reconstruct the IMF were made in 1977, when Pennisi underlined the necessity of IMF reconstruction and described a surgical method via a separate incision at the location of the new IMF. Despite providing stable results, this method had a number of disadvantages, such as additional scars, skin deficiency, and necrotic complications between the sites of mastectomy and inframammary incisions (12). This method and the similar method proposed by Ryan and Bostwick failed to gain popularity (8,10).
One of the pioneers of the use of the “internal approach” for the restoration of the IMF was Versaci, who suggested the use of advancement abdominal flaps during the expander/implant replacement procedure (11). The author noticed skin retractions in the area of the restored IMF and poorly defined creases in some cases, while other authors (12,13) noticed bulkiness in the region of the new IMF.
One of the refinements of the Versaci technique that is quite popular today was proposed by Bayati (13). This method involves expander/implant replacement, during which the anterior sheet of the capsule is totally removed while the soft tissue complex is mobilized posteriorly starting from ribs V–VI and progressing downward 8–10 cm. The mobilized thoraco-epigastric flap is then pulled upward and fixed with stitches to the periosteal layer of ribs V–VI at the level of the new IMF, which is marked pre-operatively. Compared to the methods mentioned above, this method provides the most stable results in the absence of additional scars, but is associated with bulkiness and indentations in the region of the new IMF (12).
In another method of IMF reconstruction suggested by Nava, the supposed level of the new IMF is marked using needles at the level at which the capsule is to be horizontally incised. The incision is made to the level of the superficial fascia, which is incised across the new IMF. The lower free border of the superficial fascia is sutured to the chest wall on the proposed level of the new IMF (9). This method allows a new, well-defined IMF to be reconstructed without indentations and bulkiness with long-term stable results. However, this method is also associated with the disadvantage of a blunt breast-thoracic angle (>90°), which stops or slows the process of the formation of natural ptosis with time. In the meantime, as previously mentioned Vu, it is very important to maintain the right distribution of the expansion vector of the lower pole, which allows avoiding unwanted unnatural ptosis (14).
The method suggested by our team is also performed via the “internal approach”, but is not associated with additional scarring in the field of the reconstructed breast. Similar to the other methods of IMF reconstruction, our method can be performed during the expander/implant replacement stage of two-staged expander-implant breast reconstruction. The main difference is that we suggest using the PS of the expander capsule as the “anchor” for the new IMF, which allows the creation of a well-defined IMF without any sutures inside or indentations, as well as a sharp breast-thoracic angle and stable results during long-term follow-up.
In conclusion, the IMF is an important component of the aesthetic perception of the female breast, and in cases of the destruction of this anatomical structure during previous mastectomy, it is impossible to achieve acceptable symmetry with the contralateral breast without restoration. Restoration of the IMF must become an obligatory component of breast reconstruction, because the reconstruction cannot be considered complete without this step. The restored IMF needs to be well defined and have a sharp breast-thoracic angle in order to create the impression of natural ptosis. We believe that this new technique of IMF reconstruction fulfills these requirements, and will improve the general aesthetic results of breast reconstruction surgery in the future.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the Ethics Committee of the Kazan State Medical Academy (ID: 5/12 from 03.12.2014) and conformed to the principals of the Declaration of Helsinki and its amendments. All patients provided written informed consent before participation on the study.
References
- Petit JY, Veronesi U, Luini A, et al. When mastectomy becomes inevitable: the nipple-sparing approach. Breast 2005;14:527-31. [Crossref] [PubMed]
- Wong SM, Freedman RA, Sagara Y, et al. Growing Use of Contralateral Prophylactic Mastectomy Despite no Improvement in Long-term Survival for Invasive Breast Cancer. Ann Surg 2016. [Epub ahead of print]. [PubMed]
- Cabalag MS, Rostek M, Miller GS, et al. Alloplastic adjuncts in breast reconstruction. Gland Surg 2016;5:158-73. [PubMed]
- Salgarello M, Visconti G, Barone-Adesi L. One-stage immediate breast reconstruction with implants in conservative mastectomies. Available online: .http://www.intechopen.com/books/breast-reconstruction-current-techniques/one-stage-immediate-breastreconstruction-with-implants-in-conservative-mastectomies
- Blondeel PN, Hijjawi J, Depypere H, et al. Shaping the breast in aesthetic and reconstructive breast surgery: an easy three-step principle. Plast Reconstr Surg 2009;123:455-62. [Crossref] [PubMed]
- Schuh F, Cericatto R, Bittelbrunn AC, et al. Inframammary fold reconstruction. In: Urban C, Rietjens M. editors. Oncoplastic and Reconstructive Breast Surgery. Milan: Springer; 2013:325-30.
- Pennisi VR. Making a definite inframammary fold under a reconstructed breast. Plast Reconstr Surg 1977;60:523-5. [Crossref] [PubMed]
- Ryan JJ. A lower thoracic advancement flap in breast reconstruction after mastectomy. Plast Reconstr Surg 1982;70:153-60. [Crossref] [PubMed]
- Nava M, Quattrone P, Riggio E. Focus on the breast fascial system: a new approach for inframammary fold reconstruction. Plast Reconstr Surg 1998;102:1034-45. [Crossref] [PubMed]
- Bostwick J 3rd. editor. Finishing Touches. Plastic and Reconstructive Breast Surgery. St. Louis: Quality Medical Publishing; 1990:1126.
- Versaci AD. A method of reconstructing a pendulous breast utilizing the tissue expander. Plast Reconstr Surg 1987;80:387-95. [Crossref] [PubMed]
- Pinnella JW. Creating an inframammary crease with a liposuction cannula. Plast Reconstr Surg 1989;83:925. [Crossref] [PubMed]
- Bayati S, Seckel BR. Inframammary crease ligament. Plast Reconstr Surg 1995;95:501-8. [Crossref] [PubMed]
- Vu MM, Kim JY. Current opinions on indications and algorithms for acellular dermal matrix use in primary prosthetic breast reconstruction. Gland Surg 2015;4:195-203. [PubMed]


