Short-term efficacy and safety of neoadjuvant pyrotinib plus taxanes for early HER2-positive breast cancer: a single-arm exploratory phase II trial
Highlight box
Key findings
• Neoadjuvant pyrotinib monotherapy plus chemotherapy shows promising clinical benefit in patients with human epidermal growth factor receptor 2-positive (HER2+) non-metastatic breast cancer (BC).
What is known and what is new?
• Pyrotinib-based therapy is effective for HER2+ BC at all stages.
• This study supports the use of this new neoadjuvant regimen for early or locally advanced HER2+ BC.
What is the implication, and what should change now?
• Our regime might be an alternative option for patients who have contraindications for large-molecule monoclonal antibodies or when these drugs are not available. The efficacy of pyrotinib-based neoadjuvant therapy and the optimal combination require further verification.
Introduction
Overexpression and/or amplification of human epidermal growth factor receptor 2 (HER2) is present in around 22% of early breast cancers (BC), and is associated with aggressive disease and poor prognosis (1,2). According to the National Comprehensive Cancer Network (NCCN) (3) and Chinese Society of Clinical Oncology (CSCO) (4) guidelines, neoadjuvant therapy is recommended for patients with staged II–III HER2-positive (HER2+) early or locally advanced BC (5,6). In HER2-positive breast tumors, pathological complete response (pCR) achieved by neoadjuvant regimen has been found to be correlated with improved survival outcomes, suggesting that it may serve as an early surrogate marker of clinical benefit (7-9).
HER2-targeted therapy has dramatically improved outcomes in patients with HER2+ BC and therefore constituted a standard of care. Trastuzumab (Herceptin; Roche, Basel, Switzerland), a recombinant humanized monoclonal antibody, was the first HER2 blocker and remains the cornerstone of therapy for all HER2+ BCs. Despite the efficacy of trastuzumab, drug resistance and recurrence/metastasis may occur in some patients, enhancing the need for new regimens. Small-molecule tyrosine kinase inhibitors (TKIs) have been found to suppress the growth of HER2+ BC cells through different signaling pathways, therefore representing a promising HER2 blockade overcoming drug resistance.
Pyrotinib is a new irreversible inhibitor of epidermal growth factor receptor (EGFR), HER2, and HER4. In the metastatic setting, a recent meta-analysis revealed that pyrotinib-containing regimens demonstrated considerable tumor response, survival outcome and manageable toxicity in any line of treatment for HER2+ metastatic BC (10,11). Recently, the final analysis of the phase II PANDORA trial (12) suggested that pyrotinib monotherapy plus docetaxel given in the first-line treatment was highly active with an objective response rate (ORR) of 79.7% [95% confidence interval (CI): 70.8–88.6%] among patients with HER2+ advanced BC. In a neoadjuvant setting, different pyrotinib-based chemotherapeutic regimes, mostly involving a taxane and pyrotinib plus trastuzumab, have been evaluated in some phase 2 studies which yielded high response rates (13-15). In addition, the phase 3 PHEDRA trial (16) demonstrated that neoadjuvant pyrotinib, trastuzumab, and docetaxel significantly improved the pCR rate compared with placebo, trastuzumab, and docetaxel, supporting the accelerated approval of pyrotinib for HER2+ BC in the neoadjuvant setting by China Food and Drug Administration. Nonetheless, the effect of combination of pyrotinib plus a taxane was assessed alongside with trastuzumab in the abovementioned studies. For patients with contraindications to trastuzumab or developing countries where macro-molecule monoclonal antibodies are inaccessible, evidence supporting the use of pyrotinib monotherapy plus taxanes as neoadjuvant treatment is lacking. This preliminary study aimed to explore the efficacy and safety of pyrotinib as a single HER2 blocker plus taxanes as preoperative systemic treatment for patients with early or locally advanced HER2+ BC. We present this article in accordance with the TREND reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-24-38/rc).
Methods
Study design
This single-arm, open-label phase 2 trial was implemented at The First People’s Hospital of Foshan in Guangdong, China during 1 September 2021 to 30 December 2022. The sample size of this trial was determined by the willingness of patients with early or locally advanced HER2-positive BC at The First People’s Hospital of Foshan during the study period. The trial was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki (as revised in 2013). The Ethics Committee at The First People’s Hospital of Foshan approved the protocol (approval number: 2021[113-1]), and all participants provided written informed consent. This study is registered in the Chinese Clinical Trial Registry under ChiCTR2100050870.
Participants
The key inclusion criteria were as follows: (I) patients’ age between 18 and 70 years at initial treatment; (II) patients with clinically staged II–III BC based on the criteria of the American Joint Committee on Cancer (AJCC); (III) HER2 immunohistochemical staining of 3+ and/or amplification of HER2 gene copy number by fluorescence in situ hybridization; (IV) Eastern Cooperative Oncology Group (ECOG) score of 0–1; (V) 1 or more measurable lesions according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (17); (VI) adequate bone marrow, hepatic, renal, and cardiac functions (left ventricular ejection fraction ≥55%).
The key exclusion criteria were as follows: (I) bilateral BC; (II) those with advanced BC (stage IV); (III) prior systemic therapy or radiotherapy for any malignant tumor (except for cured cervical carcinoma in situ and basal cell carcinoma); (IV) prior anticancer therapy in other clinical trials or pregnancy or lactation.
Interventions
Oral pyrotinib was administered at a dose of 400 mg once daily beginning on day 1 of taxanes and continuing through until day 28 from the final cycle of taxanes. A total of 4 cycles of docetaxel (75 mg/m2, escalating, if well tolerated, to 100 mg/m2) or nanoparticle albumin-bound (nab)-paclitaxel (260 mg/m2) were given intravenously every 3 weeks or 4 cycles of paclitaxel (80 mg/m2) given on days 1, 8, and 15 of a 21-day cycle. The choice of taxanes was determined by the treating physician and the patient. In total, patients received 4 cycles of neoadjuvant therapy of taxanes plus pyrotinib before surgery. After completion of neoadjuvant treatment, eligible patients underwent surgery and adjuvant fluorouracil/epirubicin/cyclophosphamide (FEC) therapy (3 cycles of fluorouracil 600 mg/m2 intravenously, epirubicin 90 mg/m2 intravenously, and cyclophosphamide 600 mg/m2 intravenously every 3 weeks). Thereafter, adjuvant trastuzumab was administered every 3 weeks for 1 year. Radiotherapy and standard hormone treatment for patients with positive estrogen receptor (ER) were prescribed if necessary.
Adjustment of pyrotinib dosage was adverse events (AEs)-oriented, which corresponded to a gradient of 400, 320, and 240 mg. To guarantee the drug intensity of the treatment, the maximal cumulative interruption time of pyrotinib allowed in each cycle was 14 days. Since diarrhea was expected with pyrotinib, primary prophylaxis with loperamide (4 mg) was given at the beginning of the first dose of therapy, and supplemented with 2 mg after each loose stool thereafter.
Tumor response (clinical breast examination) was assessed at every cycle. Patients underwent physical examination and ultrasound every cycle for efficacy evaluation before breast surgery. Magnetic resonance imaging (MRI) was performed at baseline and before surgery. Surgical breast specimens were assessed for pCR by the pathological department and no central review were planned.
Study endpoints and assessments
Efficacy assessment was based on pathological and clinical measurements. The primary endpoint was the total pCR (tpCR) rate, defined as absence of microscopic invasive cancer cells in both the breast and axillary lymph nodes, whereas ductal carcinoma in situ was allowed (ypT0/Tis ypN0). The secondary endpoints included: the breast pCR (bpCR) response rate defined as absence of microscopic invasive cancer cells in the breast (ypT0/Tis); the Miller-Payne (MP) system (18) was applied for analysis of histological response to study treatment; the investigator-assessed ORR which referred to the proportion of patients with complete response (CR) or partial response (PR) defined by The RECIST 1.1 criteria after completion of the neoadjuvant therapy; survival outcomes. The safety profiles were reported based on AEs graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 (https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcae_4_with_lay_terms.pdf). A sensitivity analysis was planned for efficacy in the per protocol set.
Statistical analysis
The analysis population of this study consisted of participants in the intention-to-treat (ITT) population including all patients who received at least one dose of the study treatment. A sensitivity analysis was planned for efficacy in the per protocol set. The results of this study were analyzed using descriptive statistical methods. The continuous data were presented as means ± standard deviation or medians [range], and the categorical data were presented as frequency, percentage, and 95% CIs. The software SPSS 22.0 (IBM Corp., Armonk, NY, USA) was used for statistical analyses. The two-sided P value <0.05 was considered statistically significant.
Results
Patient characteristics
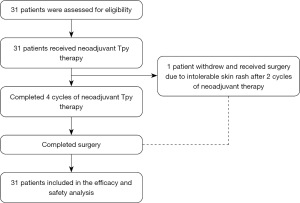
From 1 September 2021 to 30 December 2022, a total of 31 patients were enrolled (Figure 1); 30 patients completed the entire neoadjuvant therapy. After 2 cycles of neoadjuvant therapy, one patient withdrew from the trial due to intolerable skin rash, and discontinued the drugs in cycle 3. After surgery, the patient received a pertuzumab-trastuzumab containing regimen.
The baseline characteristics of the ITT population are shown in Table 1. The median age was 53 years (range, 38–65 years) with predominantly premenopausal patients (n=17, 54.8%). The proportion of patients with T2–T4 disease was 90.3% (28/31) and the proportion of patients with N1–N3 disease was 58.1% (18/31). Of the 31 cases, 20 (64.5%) showed positive ER and/or progesterone receptor (PR).
Table 1
| Characteristics | ITT population (n=31) |
|---|---|
| Age (years), median [range] | 53 [38–65] |
| Menopausal status, n (%) | |
| Premenopausal | 17 (54.8) |
| Postmenopausal | 14 (45.2) |
| Clinical tumor stage, n (%) | |
| T1 | 3 (9.7) |
| T2 | 20 (64.5) |
| T3 | 5 (16.1) |
| T4 | 3 (9.7) |
| Clinical lymph node status, n (%) | |
| N0 | 10 (32.3) |
| N1 | 14 (45.2) |
| N2 | 0 |
| N3 | 4 (12.9) |
| Nx | 3 (9.7) |
| Clinical stage, n (%) | |
| II | 20 (64.5) |
| III | 11 (35.5) |
| ECOG performance status, n (%) | |
| 0 | 31 (100.0) |
| Hormone receptor status, n (%) | |
| ER and/or PR positive | 20 (64.5) |
| ER and PR negative | 11 (35.5) |
| Histological grading, n (%) | |
| I | 1 (3.2) |
| II | 22 (71.0) |
| III | 7 (22.6) |
| Unknown | 1 (3.2) |
| Ki-67 level, n (%) | |
| <20% | 9 (29.0) |
| ≥20% | 21 (67.7) |
| Unknown | 1 (3.2) |
| Neoadjuvant regimen, n (%) | |
| Docetaxel plus pyrotinib | 8 (25.8) |
| Nab-paclitaxel plus pyrotinib | 23 (74.2) |
| Surgery, n (%) | |
| Mastectomy | 21 (67.7) |
| Breast conserving surgery | 10 (32.3) |
ITT, intention-to-treat; ECOG, Eastern Cooperative Oncology Group; ER, estrogen receptor; PR, progesterone receptor; nab-paclitaxel, nanoparticle albumin-bound paclitaxel.
Most participants used nab-paclitaxel (23/31, 74.2%) in the neoadjuvant setting and had a mastectomy (21/31, 67.7%) after preoperative systemic treatment. No patient was given a weekly paclitaxel regimen.
Efficacy
The tpCR rate after 4-cycle neoadjuvant therapy was 48.4% (95% CI: 30.8–66%) (15/31) (Table 2). Among them 8 of 20 (40.0%; 95% CI: 18.5–61.5%) tumors had positive hormone receptor (HR) and 7 of 11 (63.6%; 95% CI: 35.2–92%) tumors had negative HR (P>0.99). Postoperative pathological measurement showed that 51.6% (95% CI: 34–69.2%) (16/31) achieved bpCR. There were 2 (6.5%) and 12 (38.7%) cases with MP G1–2 and G3–4, respectively. The patient who dropped out of the trial after 2 cycles of preoperative therapy received breast conserving surgery and the pathological evaluation was MP G3. Pathological response assessment was failed in one non-pCR case because the patient underwent biopsy in another hospital.
Table 2
| Variables | ITT (n=31) | HR positive (n=20) | HR negative (n=11) | |||||
|---|---|---|---|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | |||
| tpCR | 15 | 48.4 (30.8–66.0) | 8 | 40.0 (18.5–61.5) | 7 | 63.6 (35.2–92.0) | ||
| bpCR | 16 | 51.6 (34.0–69.2) | 8 | 40.0 (18.5–61.5) | 8 | 72.7 (46.4–99.0) | ||
| MP grading# | ||||||||
| 1 or 2 | 2 | 6.5 (−2.2 to 15.2) | 2 | 10.0 (−3.1 to 23.1) | 0 | – | ||
| 3 or 4 | 12 | 38.7 (21.6–55.8) | 9 | 45.0 (23.2–66.8) | 3 | 27.3 (0.97–53.6) | ||
| ORR& | 19 | 82.6 (81.9–83.3) | – | – | – | – | ||
| CR& | 5 | 21.7 (21.0–22.4) | – | – | – | – | ||
| PR& | 14 | 60.9 (41.0–80.8) | – | – | – | – | ||
#, MP grading was not given in one HR positive patient due to absence of biopsied sample for assessment; &, ORR, CR and PR rates were based on 23 patients who had complete MRI data for assessment. ITT, intention-to-treat; HR, hormone receptor; CI, confidence interval; tpCR, total pathological complete response; bpCR, breast pathological complete response; MP, Miller-Payne; ORR, objective remission rate; CR, complete remission; PR, partial remission; MRI, magnetic resonance imaging.
Regarding clinical response to neoadjuvant therapy based on MRI, 23 of 31 patients (74.2%) had complete imaging results at baseline and 4 cycles (Table 2). There were 4 patients (17.4%) who presented with stable disease (SD) and no tumor progression occurred.
All 31 patients (100%) experienced treatment-related AEs, the majority of which were grade 1–2. A large number of AEs were due to the taxane. AEs with an incidence of ≥10% are listed in Table 3. The most frequent AEs were diarrhea (100%), fatigue (64.5%), loss of appetite (61.3%), abdominal pain (54.8%), and hand and foot numbness (54.8%). Grade ≥3 AEs included diarrhea (n=2, 6.5%), hand and foot numbness (n=1, 3.2%), loss of appetite (n=1, 3.2%), and skin rash (n=1, 3.2%). All patients with grade 3 AEs recovered to grade 2 or below before the following cycle of therapy; no dose adjustment or treatment delayed was required. Only one patient experienced uncontrollable skin rash and left the trial after completion of the second cycle of neoadjuvant therapy.
Table 3
| Adverse event | Patients (n=31), n (%) | ||
|---|---|---|---|
| Any grade | Grade 3 | Grade 4 | |
| Diarrhea | 31 (100.0) | 2 (6.5) | 0 |
| Neutrophil count decreased | 11 (35.5) | 0 | 0 |
| WBC count decreased | 11 (35.5) | 0 | 0 |
| Hand and foot numbness | 17 (54.8) | 1 (3.2) | 0 |
| Fatigue | 20 (64.5) | 0 | 0 |
| Vomiting | 11 (35.5) | 0 | 0 |
| Nausea | 15 (48.4) | 0 | 0 |
| Oral mucositis | 10 (32.3) | 0 | 0 |
| Loss of appetite | 19 (61.3) | 1 (3.2) | 0 |
| Increased transaminase | 8 (25.8) | 0 | 0 |
| Skin rash | 9 (29.0) | 0 | 1 (3.2) |
| Abdominal pain | 17 (54.8) | 0 | 0 |
| Cardia dysfunction | 0 | 0 | 0 |
| Death related to treatment | 0 | 0 | 0 |
WBC, white blood cell.
Discussion
This study investigated the efficacy and safety of neoadjuvant pyrotinib monotherapy plus taxanes in patients early or locally advanced HER2+ BC. The data showed encouraging tpCR and bpCR rates of 48.4% (95% CI: 30.8–66%) and 51.6% (95% CI: 34–69.2%) (16/31), respectively.
The HER2-targeted drugs are composed of large-molecule monoclonal antibodies (including trastuzumab and pertuzumab) and small-molecule TKIs (such as lapatinib, neratinib and pyrotinib). In some randomized controlled trials (NeoALTTO, Neosphere, PEONY, and PHEDRA), neoadjuvant trastuzumab monotherapy plus taxanes exhibited a tpCR rate of 21.5–27.6% (19-22). In the subgroup of patients receiving pertuzumab plus docetaxel in the Neosphere trial (20), the tpCR rate was 29%.
In contrast to anti-HER2 monoclonal antibodies, TKIs compete with tyrosine kinase coupling with the HER2 intracellular kinase domain and block downstream signaling pathways. TKIs have the advantages of blocking multiple targets, oral administration, and decreased cardiac toxicity (23). The NeoALTTO trial (19) showed that the pCR rate of lapatinib monotherapy arm was 24.7% (95% CI: 18.1–32.3%). In the NSABP FB-7 trial, the numerical pCR rate in the single-targeted therapy with neratinib arm was 33% (24). In studies assessing lapatinib/neratinib-containing neoadjuvant therapies, the pCR rates of the TKI groups were 24–53.2% (19,25,26). In these studies, the pCR rates were comparable between the lapatinib/neratinib group and the trastuzumab group. Furthermore, addition of a TKI into a trastuzumab-based regime in a neoadjuvant setting has resulted in a favorable pCR rate of 51–62% with tolerable toxicity (24,27,28).
In this present exploratory study, the tpCR and bpCR rates of pyrotinib plus taxanes were encouraging with 48.4% (95% CI: 30.8–66%) and 51.6% (95% CI: 34.0–69.2%), respectively. Anti-tumor activity appeared to be among the highest observed activity among the abovementioned mono-targeted anti-HER2 regimen.
Regardless of the different clinical settings between the studies, the role of pyrotinib as a component of neoadjuvant therapy for HER2-positive BC deserves further research. Recently, various combinations of pyrotinib plus chemotherapy for HER2+ BC in the neoadjuvant setting have been increasingly explored. Several phase II trials showed that dual-target therapy with trastuzumab and pyrotinib plus neoadjuvant chemotherapy (NAC) exhibited pCR rates between 51.6% and 73.7% (13,15,29,30). A real-world analysis found that the tpCR rate of pyrotinib-containing neoadjuvant therapy was 48.5%, respectively (28). An observational study comparing pyrotinib or pertuzumab plus trastuzumab in combination with NAC revealed that the tpCR and bpCR rates were 64.5% vs. 54.0% and 76.2% vs. 58.0%, albeit insignificantly (31). Incorporating the results of the present prospective study, pyrotinib-based neoadjuvant treatment shows a promising effectiveness for patients with early or locally advanced HER2+ BC.
As expected, pyrotinib-related diarrhea was the most frequent AE, whereas the overall safety profile in the present study was acceptable. The incidence of grade ≥3 diarrhea in this study was much lower than that of previously reported pyrotinib-containing neoadjuvant therapy (6.5% vs. 18.2–64.5%) (13-15,29). The use of a single HER2-targeted agent and a single chemo-drug in the neoadjuvant setting might have contributed to the low incidence of severe diarrhea. Moreover, primary prophylaxis with loperamide may also be helpful. No evidence showed that pyrotinib is correlated with cardiotoxicity. Definitively, the overall tolerability profile of pyrotinib regimen remained favorable allowing further development.
There were some limitations in this study that should be mentioned. Firstly, the sample size was limited and prolonged follow-up is required to verify the clinical benefit. The absence of a control group limits the level of evidence. Moreover, differences in defining pCR as well as in the chemotherapeutic regimes adopted in each trial may result in variations regarding pCR rates. Nevertheless, we aimed to provide supplementary data on pyrotinib-containing neoadjuvant therapy in HER2+ breast cancer.
Conclusions
In conclusion, our study suggested that the association pyrotinib plus taxanes is a promising neoadjuvant regimen in patients with early or locally advanced HER2+ BC. Pyrotinib should be considered in the treatment of early breast cancer. This combination in our study might provide an alternative option for patients who cannot receive large-molecule monoclonal antibody treatment or when these drugs are not available. However, the reported efficacy of pyrotinib-based neoadjuvant therapy should trigger further assessments. The investigation of the optimal chemo-partner, the activity of a doublet regimen combining pyrotinib and trastuzumab worth further trials.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-24-38/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-24-38/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-24-38/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-24-38/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki (as revised in 2013). The Ethics Committee at The First People’s Hospital of Foshan approved the protocol (approval number: 2021[113-1]), and all participants provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zell JA, Tsang WY, Taylor TH, et al. Prognostic impact of human epidermal growth factor-like receptor 2 and hormone receptor status in inflammatory breast cancer (IBC): analysis of 2,014 IBC patient cases from the California Cancer Registry. Breast Cancer Res 2009;11:R9. [Crossref] [PubMed]
- Ross JS, Slodkowska EA, Symmans WF, et al. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist 2009;14:320-68. [Crossref] [PubMed]
- Gradishar WJ, Moran MS, Abraham J, et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022;20:691-722. [Crossref] [PubMed]
- Huang X, Yin YM. Updates of Chinese Society of Clinical Oncology (CSCO) guideline for breast cancer in 2018. Zhonghua Yi Xue Za Zhi 2018;98:1213-7. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology-Invasive Breast Cancer (2022 Version II). Available online: http://www.nccn.org
Breast Cancer Guidelines of Chinese Society of Clinical Oncology (CSCO) 2021 ). Available online: http://www.csco.org.cn/- Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 2010;375:377-84. [Crossref] [PubMed]
- Untch M, Fasching PA, Konecny GE, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol 2011;29:3351-7. [Crossref] [PubMed]
- Pivot X, Cox DG. A new era for treatment development in HER2-positive breast cancer. Lancet Oncol 2018;19:160-2. [Crossref] [PubMed]
- Kioutchoukova I, Lucke-Wold BP. Pyrotinib as a therapeutic for HER2-positive breast cancer. Transl Cancer Res 2023;12:1376-9. [Crossref] [PubMed]
- Hu W, Yang J, Zhang Z, et al. Pyrotinib for HER2-positive metastatic breast cancer: a systematic review and meta-analysis. Transl Cancer Res 2023;12:247-56. [Crossref] [PubMed]
- Zheng Y, Cao WM, Shao X, et al. Pyrotinib plus docetaxel as first-line treatment for HER2-positive metastatic breast cancer: the PANDORA phase II trial. Nat Commun 2023;14:8314. [Crossref] [PubMed]
- Xuhong J, Qi X, Tang P, et al. Neoadjuvant Pyrotinib plus Trastuzumab and Chemotherapy for Stage I-III HER2-Positive Breast Cancer: A Phase II Clinical Trial. Oncologist 2020;25:e1909-20. [Crossref] [PubMed]
- Zhong X, He P, Chen J, et al. Neoadjuvant pyrotinib plus trastuzumab and nab-paclitaxel for HER2-positive early or locally advanced breast cancer: an exploratory phase II trial. Gland Surg 2022;11:216-25. [Crossref] [PubMed]
- Liu Z, Wang C, Chen X, et al. Pathological response and predictive role of tumour-infiltrating lymphocytes in HER2-positive early breast cancer treated with neoadjuvant pyrotinib plus trastuzumab and chemotherapy (Panphila): a multicentre phase 2 trial. Eur J Cancer 2022;165:157-68. [Crossref] [PubMed]
- Wu J, Jiang Z, Liu Z, et al. Neoadjuvant pyrotinib, trastuzumab, and docetaxel for HER2-positive breast cancer (PHEDRA): a double-blind, randomized phase 3 trial. BMC Med 2022;20:498. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Ogston KN, Miller ID, Payne S, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast 2003;12:320-7. [Crossref] [PubMed]
- Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 2012;379:633-40. [Crossref] [PubMed]
- Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:25-32. [Crossref] [PubMed]
- Shao Z, Pang D, Yang H, et al. Efficacy, Safety, and Tolerability of Pertuzumab, Trastuzumab, and Docetaxel for Patients With Early or Locally Advanced ERBB2-Positive Breast Cancer in Asia: The PEONY Phase 3 Randomized Clinical Trial. JAMA Oncol 2020;6:e193692. [Crossref] [PubMed]
- Wu J, Liu Z, Yang H, et al. Abstract PD8-08: Pyrotinib in combination with trastuzumab and docetaxel as neoadjuvant treatment for HER2-positive early or locally advanced breast cancer (PHEDRA): A randomized, double-blind, multicenter, phase 3 study. Cancer Res 2022;82:PD8-08.
- Wang J, Xu B. Targeted therapeutic options and future perspectives for HER2-positive breast cancer. Signal Transduct Target Ther 2019;4:34. [Crossref] [PubMed]
- Jacobs SA, Robidoux A, Abraham J, et al. NSABP FB-7: a phase II randomized neoadjuvant trial with paclitaxel + trastuzumab and/or neratinib followed by chemotherapy and postoperative trastuzumab in HER2(+) breast cancer. Breast Cancer Res 2019;21:133. [Crossref] [PubMed]
- Swain SM, Tang G, Lucas PC, et al. Pathologic complete response and outcomes by intrinsic subtypes in NSABP B-41, a randomized neoadjuvant trial of chemotherapy with trastuzumab, lapatinib, or the combination. Breast Cancer Res Treat 2019;178:389-99. [Crossref] [PubMed]
- Wang C, Chen J, Xu X, et al. Dual HER2 Blockade in Neoadjuvant Treatment of HER2+ Breast Cancer: A Meta-Analysis and Review. Technol Cancer Res Treat 2020;19:1533033820960721. [Crossref] [PubMed]
- Hurvitz SA, Caswell-Jin JL, McNamara KL, et al. Pathologic and molecular responses to neoadjuvant trastuzumab and/or lapatinib from a phase II randomized trial in HER2-positive breast cancer (TRIO-US B07). Nat Commun 2020;11:5824. [Crossref] [PubMed]
- Mao X, Lv P, Gong Y, et al. Pyrotinib-Containing Neoadjuvant Therapy in Patients With HER2-Positive Breast Cancer: A Multicenter Retrospective Analysis. Front Oncol 2022;12:855512. [Crossref] [PubMed]
- Yao DS, Wang W, Chang JY, et al. Neoadjuvant pyrotinib plus nab-paclitaxel, doxorubicin, and cyclophosphamide for HER2-positive locally advanced breast cancer: a retrospective case-series study. Gland Surg 2021;10:3362-8. [Crossref] [PubMed]
- He L, Zhang F, Ma Y, et al. Pathological Complete Response from Pyrotinib Combined with Trastuzumab, Paclitaxel and Cisplatin in a Postpartum Woman with HER2-Positive Locally Advanced Breast Cancer: A Case Report. Onco Targets Ther 2020;13:8749-56. [Crossref] [PubMed]
- Li Q, Wang Y, Zhu M, et al. Clinical observation of neoadjuvant chemotherapy with pyrotinib plus trastuzumab in HER2-positive breast cancer: a cohort study. Gland Surg 2021;10:3389-402. [Crossref] [PubMed]