Innovative solutions in bariatric surgery
Introduction
Obesity, defined as a BMI of ≥30 kg/m2, has recognized as an epidemic of XXI century (1). It shortens patients’ life, decreases its quality, often leads to other health problems, such as type 2 diabetes mellitus, hypertension, coronary heart disease or dyslipidemia. Despite numerous consensus meetings we still do not have clear guidelines concerning the treatment of obesity. Even though the most effective way to treat obesity is bariatric surgery, it is far from being a perfect solution. With efficacy of 80% [measured as excess weight loss (EWL) of at least 50% during 5 years after surgery] and mortality 0.2–0.4% (2). The ideal procedure should be safe, effective, durable, repeatable, reversible, minimally-invasive and cost-effective. So far such solution has not been found yet. Each year a new device or procedure is being introduced, making the field of obesity surgery the most rapidly developing world health market. Diabetes control is another main target for new procedures and the branch of “metabolic surgery” is currently becoming more important than “bariatric surgery”.
Being the youngest subspecialty of surgery, bariatric surgery is quickly advancing on top in terms of number of procedures performed worldwide. The history of bariatric surgery has started in 1950’s. From resection of small intestine it evolved through gastric bypass, biliopancreatic diversion with duodenal switch and vertical banded gastroplasty towards, adjustable gastric banding and sleeve gastrectomy (3). In the beginning bariatric surgery was an open surgery. Time has changed and the minimally invasive techniques have improved. Nowadays, bariatric procedures are carried out laparoscopically. First laparoscopic gastric bypass was published in 1994 by Wittgrove and co-authors (4). This procedure is still the first of second most frequently performed one and is considered a standard, to which other methods are compared (5). Laparoscopic sleeve gastrectomy, the most commonly used bariatric procedure in the US (5), was firstly reported as a primary operation in 2003 (6).
The patients that are qualified to the surgery should be adults with a body mass index (BMI) ≥40 kg/m2 without comorbid illness or with a BMI 35.0 to 39.9 kg/m2 with at least one serious comorbidity, such as type 2 diabetes, hypertension, hyperlipidemia and some others. There are also some contraindications, such as bulimia nervosa, untreated major depression, drug or alcohol abuse, coagulopathy or inability to understand or respect postoperative recommendations (7).
In this paper we are presenting new modifications and innovative procedures in bariatric surgery based on our clinical experience and current literature. We decided to divide our paper into four chapters: innovative modifications of standard techniques; innovative restrictive solutions; innovative malabsorbtive solutions and innovations beyond the basic options.
Innovative modifications of standard techniques
New tools
Thanks to continuous technological development, current laparoscopic surgery is becoming safer and faster. Recent advances in ultra-high resolution and 3D imaging allow better visualization of operative field in minimally invasive surgery. New instruments for advanced hemostasis, based on bipolar or harmonic energy, or their combination are becoming faster and more efficient. New stapling devices with engine and microprocessor are becoming sensitive to tissue resistance, allowing for better adapted staple height and more gentle progress in stapling and cutting tissues. Illumination prevents stapling and cutting through newly developed gastric catheters. Better devices to close mesenteric openings and wounds after laparoscopy help surgeons prevent herniation and wound bleeding. Thus safety of the patient is increasing. All at some additional expense. A question about cost-effectiveness arises, but so far remains unanswered.
New approaches
In aim to minimize the number of incisions, some surgeons perform bariatric operations through single incisions, leaving behind only one scar (8). Personally we are not sure if this is a real progress. From our experience, such procedures take longer and are more difficult than “standard” laparoscopic procedures. Is additional risk and inconvenience worth a single scar? We are not certain and others share this attitude (9). Some other surgeons limit the number of incisions to three. Again, we have performed sleeve gastrectomies using only 3 incisions. However, the visualization of most crucial anatomical landmarks, like left crus of diaphragm becomes more difficult and can pose additional risk of bleeding and thermal injury. Moreover it is possible only in selected patients, definitely not superobese patients. What is worth attempting, in our view, is to limit the size of the trocars, e.g., using 5 mm trocar instead of 10 or 13 mm, thus decreasing wound complications.
Enhanced recovery after surgery (ERAS)
There is no doubt that enhanced recovery after surgery is a safe, economic and beneficial protocol. There are several reports demonstrating the advantage of ERAS versus standard care after laparoscopic sleeve gastrectomy. Lemanu et al. (10) in a randomized clinical trial proved that most of the patients can be discharged from the hospital safely in the first postoperative day. Similar studies are available for laparoscopic Roux-en-Y gastric bypass (11). Furthermore, thanks to the ERAS protocol, the cost of treatment can be cut down, proving cost-effectiveness of ERAS strategy (12). In our experience ERAS protocol is a safe and well tolerated solution. Its additional benefit is better quality of life of patients in the perioperative period. Every bariatric patient has to be informed, however, that in case of any abdominal pain or other alerting symptom, immediate contact with the hospital staff and possibly re-admission to the hospital is necessary.
Innovative restrictive solutions
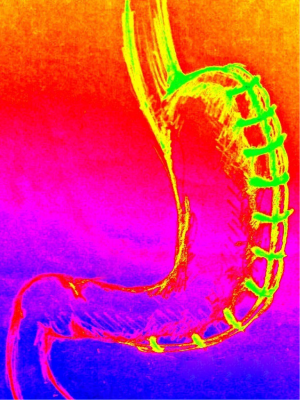
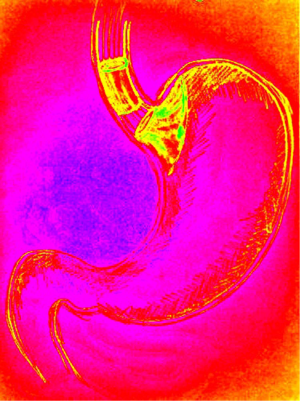
Gastric plication (Figure 1)
This emerging restrictive laparoscopic technique could be compared with laparoscopic sleeve gastrectomy. The procedure starts from dividing the greater curvature of the stomach from the omentum by using the electrocautery device. Afterwards the plication is carried out by using interrupted or running sutures starting 1–2 cm below the His angle in the direction to the pylorus. The method seems relatively easy and safe. However the effect on EWL, promising after 1 year—46% is lost with time, after 3 years—20.5% only (13).
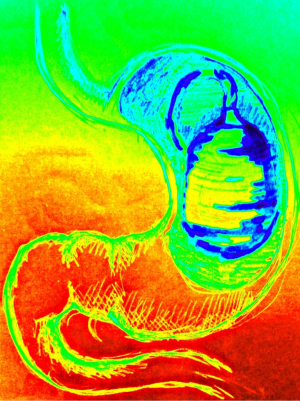
Intragastric balloon therapy (Figure 2)
Intragastric balloon is a purely restrictive method of obesity treatment. Two of the devices have been approved by The Food and Drug Administration (FDA) for obese patients with BMI 30–40 kg/m2: Orbera Bioenterics Intragastric Balloon and ReShape Dual Balloon. Both are inserted and removed during gastroscopy and filled with 400 to 700 mL sterile saline and about ten millimeters of blue methylene (if the balloon is broken, the blue methylene changes the color in green, that can be visualised in the urine). After 6 months the balloon should be removed. The method seems to be an easy and safe, however the effect on excess weight loss is limited—14–32% at 6 months (14,15). Intra-gastric balloon is recommended for temporary use for bariatric patients with high BMI as a bridge to surgery. Complications of intragastric balloon include aspiration, migration into small intestine with possible bowel obstruction, or gastric perforation. The last complication has been observed especially after prolonged use of the device (15).
Endoscopic vertical gastroplasty
Vertical banded gastroplasty is at present a rarely performed restrictive procedure. However, its endoluminal equivalent, called endoscopic vertical gastroplasty is gaining interest as an endoscopic revision procedure performed for weight regain after gastric bypass. The efficacy of the method measured as EWL varies between 27% and 58% after one year. The limitation of the method is its technical difficulty and questioned durability (16,17).
Endoscopic restrictive implant
Some new endoluminal devices mimicking a gastric band have been developed, including Trans-oral Endoscopic Restrictive Implant System (TERIS). Early results showed EWL of 28% (18) with some complications during first implantations, that were eliminated after technical improvements. Long-term results are necessary to confirm safety and efficacy of this endoluminal system.
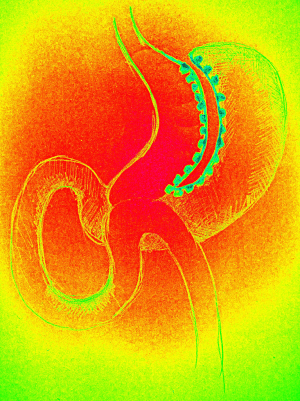
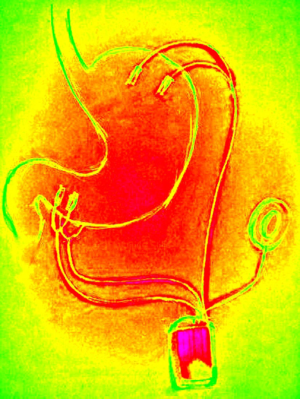
Percutaneous gastric emptying (Figure 3)
Aspire Assist is a percutaneous gastrostomy, that can be used to aspirate the chyme from the stomach. The device can be inserted endoscopically under sedation and local anesthesia. A 20F or 24F tube is placed in the greater curvature of the stomach over the wire. The device should be used 20 min after each meal and the ingested food can be aspirated to the toilet. There is a single pilot study (19), reporting EWL of 49% after 1 year and 56% after 2 years. No serious adverse effects have been reported (19). The device has FDA registration, despite its controversial way of action.
Innovative malabsorptive solutions
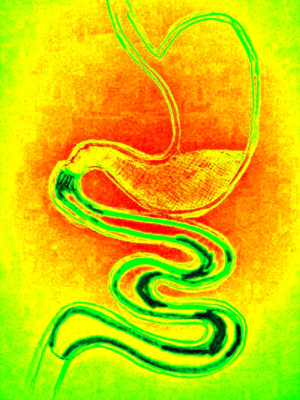
Omega-loop gastric bypass (Figure 4)
This procedure was popularized and described in a group of 1,274 patients by Rutledge (20). Operative technique differs from standard Roux-en-Y by creating a longer, but narrow gastric pouch. The stomach is divided between the antrum and the body over a gastric tube. A single gastrojejunal anastomosis is done, by taking the jejunum 200 cm from the Treitz ligament. Comparing it with Roux-en-Y Gastric Bypass, it is somewhat easier to perform and the time of surgery is shorter. The effect on EWL is comparable to Roux-en-Y gastric bypass (71.5% after 5 years) and diabetes cure rate is pretty high (21).
Although the surgery seems to be easy, safe, with a low complication rate, it can cause severe gastroesophageal reflux symptoms, requiring conversion to standard Roux-en-Y gastric bypass (21).
Banded gastric-bypass
Gastric bypass remains one of two most frequently performed bariatric procedures. One of modifications developed to increase the efficacy of this procedure was to add a Silastic ring around the gastric pouch. Early results of a randomized study show greater weight loss at the expense of three times more frequent vomiting after banded vs. not-banded gastric bypass. The quality of life was comparable during 2 years observation (22). However, the band is a foreign body and may migrate into the lumen of the gastric pouch, causing acute abdominal symptoms (23).
Duodenojejunal bypass sleeve (Figure 5)
Duodenojejunal bypass sleeve was designed to mimic the incretin effect of Roux-en-Y gastric bypass, by bypassing the duodenum. The device, called EndoBarrier is a fluoropolymer 60 cm long sleeve that is inserted endoscopically in the duodenum and ends in the proximal jejunum. The basic principle of this approach is to stop the absorption of the ingested food in the proximal small intestine. What is more biliopancreatic fluid does not mix with food until the jejunum. It is quite a new technique, with sparse literature reports and only short-term results are known (24). There is a problem of patient intolerance, so that most of the devices had to be shortly removed. To date, the device is not approved by The Food and Drug Administration. Another sleeve, extending from the gastric cardia to the jejunum is called ValenTx and has 120 cm. Its early results are quite impressive, reaching 40% of EWL after 3 months. The disadvantages are few and can be successfully treated with device removal (24).
Innovations beyond the basic options
Gastric neuromodulation (Figure 6)
Another approach, that is also used for treatment of bariatric patients is based on the phenomenon of modulation of vagal nerves activity. Interest in this method of treatment started with the observed weight loss after vagotomy. Unfortunately this effect was not durable and after a few months the patients regained weight. Contemporary devices use carefully defined specifications of electric current to inhibit vagal nerve activity. In the setting of intermittent inhibition (during the day), the vagal nerves can recover during the night and the effects of the procedure last much longer than after permanent vagotomy. This way of treatment allowed for better control of blood glucose in diabetic patients. There are two main systems: Tantalus used mainly in Europe and the Maestro System, registered with FDA. The approach is based on applying the device on the anterior and posterior vagus nerves by during laparoscopy. The Maestro System blocks efferent and afferent signals from the vagus nerve, leading to stimulation of neurohormonal pathways, which can increase the feeling of satiety. To implant the device, dissection of the diaphragm is necessary. The mean EWL after 12 months is 24%. The reversibility of this procedure is its important advantage. On the other hand, patients after this kind of surgery may have diaphragmatic hernia and may feel discomfort in the upper part of the stomach (25).
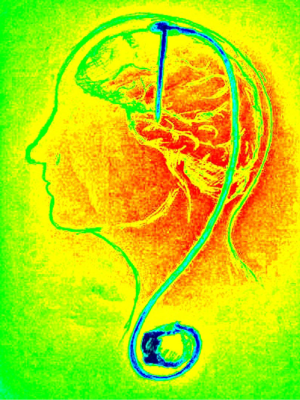
Deep brain neuromodulation (Figure 7)
Deep brain stimulation has been successfully used to treat Parkinson’s disease and some neuropsychiatric disorders. Since neurophysiological studies have shown that nucleus accumbens is responsible for rewarding (happy feeling) after eating, it became a target of deep brain stimulation (26). A report on a single patient with bilateral implantation of stimulating electrodes into nucleus accumbens in a 19-year-old girl, suffering from obesity as a result of hypothalamus insufficiency after craniopharyngioma removal. The patient experienced a substantial subjective decrease in appetite and weight loss from 151.4 to 132 kg after 3 months and 138 kg after 14 months of follow-up. At the same time, neuropsychological tests results were intact. The technical problems with stimulating device in this patient were uncontrolled periods of switching the stimulator off, which needed correction during follow-up visits (27). Other experiences with deep brain stimulation for obesity are very limited (26). This modality of treatment, even though present results are modest, seems very promising especially in the setting of failed or contraindicated bariatric procedures (e.g., in Prader-Willi syndrome). Many technical problems (electrical settings of the stimulator and its surveillance) remain to be settled.
Gastric artery embolization
In search of new alternatives to treat obesity without surgery, endovascular embolization of left gastric artery has been performed in a limited number of patients. Previous animal studies have demonstrated a decreased ghrelin production after the procedure. Apparently the procedure is safe, however the data about its efficacy is still awaited (28).
Esophageal stent (Figure 8)
An interest in esophageal stents to treat obesity was based on the observation of a single patient with a stent inserted due to gastrojejunostomy stenosis after gastric bypass. The stent had an upside-down funnel shape and after endoluminal introduction and suturing to the esophagus and stomach, it kept gastric fundus stretched. Dr. Randal Baker observed that despite eliminating the intolerance to solid food, the patients lost weight after stent insertion. The most probable reason for the stent efficacy is stretching of the gastric fundus, imitating gastric distension after the meal. Unpublished early results of “Full Sense DeviceTM” stents are very good and reach 75% EWL after 6 months. Still official information is lacking.
Conclusions
Nowadays bariatric surgery is the most rapidly growing branch of surgery in the world. Number of obesity surgeries performing today is increasing. The qualification to the surgery should be considered individually. New endoscopic devices are emerging, such as intragastric balloon, endoscopic vertical gastroplasty or duodenal-jejunal bypass sleeve or aspire assist system, but the long-term results are not satisfactory. Endoluminal methods are an intriguing strategy for weight regain after bariatric surgery, however, they require highly skilled and experienced endoscopists to obtain good results. Many devices are no longer commercially available, due to long validation procedures. New promising technologies are emerging on the horizon, including neuromodulation and esophageal stents. They must be vigorously studied and improved before implementation in the clinical practice.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i-xii, 1-253. [PubMed]
- Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014;63:2985-3023. [Crossref] [PubMed]
- Celio AC, Pories WJ. A History of Bariatric Surgery: The Maturation of a Medical Discipline. Surg Clin North Am 2016;96:655-67. [Crossref] [PubMed]
- Wittgrove AC, Clark GW, Tremblay LJ. Laparoscopic Gastric Bypass, Roux-en-Y: Preliminary Report of Five Cases. Obes Surg 1994;4:353-357. [Crossref] [PubMed]
- Azagury DE, Morton JM. Bariatric Surgery: Overview of Procedures and Outcomes. Endocrinol Metab Clin North Am 2016;45:647-56. [Crossref] [PubMed]
- Coleman MH, Awad ZT, Pomp A, et al. Laparoscopic closure of the Petersen mesenteric defect. Obes Surg 2006;16:770-2. [Crossref] [PubMed]
- De Luca M, Angrisani L, Himpens J, et al. Indications for Surgery for Obesity and Weight-Related Diseases: Position Statements from the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO). Obes Surg 2016;26:1659-96. [Crossref] [PubMed]
- Gaillard M, Tranchart H, Lainas P, et al. Single-port laparoscopic sleeve gastrectomy as a routine procedure in 1000 patients. Surg Obes Relat Dis 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Shah AS, Campos GM. Single-incision laparoscopic sleeve gastrectomy: Is it worth it? Surg Obes Relat Dis 2016;12:935-6. [Crossref] [PubMed]
- Lemanu DP, Singh PP, Berridge K, et al. Randomized clinical trial of enhanced recovery versus standard care after laparoscopic sleeve gastrectomy. Br J Surg 2013;100:482-9. [Crossref] [PubMed]
- Hahl T, Peromaa-Haavisto P, Tarkiainen P, et al. Outcome of Laparoscopic Gastric Bypass (LRYGB) with a Program for Enhanced Recovery After Surgery (ERAS). Obes Surg 2016;26:505-11. [Crossref] [PubMed]
- Simonelli V, Goergen M, Orlando GG, et al. Fast-Track in Bariatric and Metabolic Surgery: Feasibility and Cost Analysis Through a Matched-Cohort Study in a Single Centre. Obes Surg 2016;26:1970-7. [Crossref] [PubMed]
- Grubnik VV, Ospanov OB, Namaeva KA, et al. Randomized controlled trial comparing laparoscopic greater curvature plication versus laparoscopic sleeve gastrectomy. Surg Endosc 2016;30:2186-91. [Crossref] [PubMed]
- Dastis NS, François E, Deviere J, et al. Intragastric balloon for weight loss: results in 100 individuals followed for at least 2.5 years. Endoscopy 2009;41:575-80. [Crossref] [PubMed]
- Moura D, Oliveira J, De Moura EG, et al. Effectiveness of intragastric balloon for obesity: A systematic review and meta-analysis based on randomized control trials. Surg Obes Relat Dis 2016;12:420-9. [Crossref] [PubMed]
- Fogel R, De Fogel J, Bonilla Y, et al. Clinical experience of transoral suturing for an endoluminal vertical gastroplasty: 1-year follow-up in 64 patients. Gastrointest Endosc 2008;68:51-8. [Crossref] [PubMed]
- Brethauer SA, Chand B, Schauer PR, et al. Transoral gastric volume reduction as intervention for weight management: 12-month follow-up of TRIM trial. Surg Obes Relat Dis 2012;8:296-303. [Crossref] [PubMed]
- de Jong K, Mathus-Vliegen EM, Veldhuyzen EA, et al. Short-term safety and efficacy of the Trans-oral Endoscopic Restrictive Implant System for the treatment of obesity. Gastrointest Endosc 2010;72:497-504. [Crossref] [PubMed]
- Sullivan S, Stein R, Jonnalagadda S, et al. Aspiration therapy leads to weight loss in obese subjects: a pilot study. Gastroenterology 2013;145:1245-52.e1-5.
- Rutledge R. The mini-gastric bypass: experience with the first 1,274 cases. Obes Surg 2001;11:276-80. [Crossref] [PubMed]
- Bruzzi M, Rau C, Voron T, et al. Single anastomosis or mini-gastric bypass: long-term results and quality of life after a 5-year follow-up. Surg Obes Relat Dis 2015;11:321-6. [Crossref] [PubMed]
- Rasera I Jr, Coelho TH, Ravelli MN, et al. A Comparative, Prospective and Randomized Evaluation of Roux-en-Y Gastric Bypass With and Without the Silastic Ring: A 2-Year Follow Up Preliminary Report on Weight Loss and Quality of Life. Obes Surg 2016;26:762-8. [Crossref] [PubMed]
- Marins Campos J, Moon RC, Magalhães Neto GE, et al. Endoscopic treatment of food intolerance after a banded gastric bypass: inducing band erosion for removal using a plastic stent. Endoscopy 2016;48:516-20. [Crossref] [PubMed]
- Sandler BJ, Rumbaut R, Swain CP, et al. Human experience with an endoluminal, endoscopic, gastrojejunal bypass sleeve. Surg Endosc 2011;25:3028-33. [Crossref] [PubMed]
- Ikramuddin S, Blackstone RP, Brancatisano A, et al. Effect of reversible intermittent intra-abdominal vagal nerve blockade on morbid obesity: the ReCharge randomized clinical trial. JAMA 2014;312:915-22. [Crossref] [PubMed]
- Franco R, Fonoff ET, Alvarenga P, et al. DBS for Obesity. Brain Sci 2016.6. [PubMed]
- Harat M, Rudaś M, Zieliński P, et al. Nucleus accumbens stimulation in pathological obesity. Neurol Neurochir Pol 2016;50:207-10. [Crossref] [PubMed]
- Anton K, Rahman T, Bhanushali A, et al. Bariatric Left Gastric Artery Embolization for the Treatment of Obesity: A Review of Gut Hormone Involvement in Energy Homeostasis. AJR Am J Roentgenol 2016;206:202-10. [Crossref] [PubMed]