Intraoperative neural monitoring in thyroid surgery: lessons learned from animal studies
Introduction
Intraoperative recurrent laryngeal nerve (RLN) injury is a major source of morbidity from thyroid surgery. Unilateral injury can cause voice change and aspiration whereas bilateral injury can cause stridor and acute airway obstruction. Intraoperative RLN injury is a leading cause of medico-legal litigation after thyroid surgery (1-3). In the past decade, intraoperative neural monitoring (IONM) has gained wide acceptance as an adjunct technique for localizing and identifying the RLN and external branch of the superior laryngeal nerve (EBSLN) (4,5), for detecting anatomic variations (6-8), for detecting and elucidating mechanisms of nerve injury, and for predicting the outcome of vocal cord function (9-14). Although it does not replace knowledge of surgical anatomy or excellent surgical technique, IONM adds a new functional dynamic to surgery because it empowers surgeons beyond what is available to them through visual information alone.
In recent years, many animal studies have attempted to solve common pitfalls of IONM and to investigate new applications. The use of animal models is also important for providing the education and training needed to master clinical skills. Specifically, the combination of IONM technology and animal models is a valuable tool for studying the pathophysiology of RLN injury. Therefore, this article reviewed the literature to determine the status of animal models relevant to IONM.
Animal models for IONM research
Animal models have accelerated research and development of IONM. In the past decade, new applications of IONM in thyroid surgery have been described in animal studies, most of which have used medium-sized experimental animals such as canine/dog (15-17) and porcine/swine/mini-pig (13,18-37).
The dog larynx has been used as an animal model for more than two centuries of phonatory research. Dog models of laryngeal function and the RLN are well-established and closely mimic the anatomy, size and physiology found in humans. Canine neuroanatomy is also very similar to that in humans (15,16,38,39). After using a canine model for IONM research for several years, the Massachusetts Eye and Ear Infirmary (MEEI) group reported that the canine model accurately mimics the human RLN and laryngeal physiology and that studies of canine RLN injury help surgeons to predict nerve function and make decisions during thyroid surgery (15-17).
The oldest animal model used in RLN research is the porcine model. During the second century A.D., Galen was the first to define the precise course and function of the RLN. His experiments in live pigs found that alterations in a section of one RLN changed the tone of squealing and that alterations in sections of both RLNs induced respiratory crisis (40,41). Another reason for the widespread use of porcine models in IONM research is their high anatomical and physiological similarity to humans (42,43). Additionally, the pig is a widely available and relatively inexpensive experimental animal. Another advantage is its medium size, which enables easy handling (44). After using the porcine model for IONM research for many years and in many studies, the Kaohsiung Medical University (KMU) group considers the porcine model a reliable and reproducible model for evaluating electrophysiologic correlates of electromyography (EMG) during IONM (Figure 1) (13,18-26).
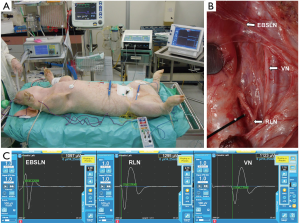
Lessons learned from animal studies
Experimental studies of new applications of IONM and its common pitfalls have addressed several research issues: (I) safety considerations of electrical stimulation (18,27); (II) characteristics of laryngeal EMG (L-EMG) responses evoked by different stimuli/recordings (16,24); (III) anesthetic and EMG tube issues (19,20,25,30,31); (IV) new techniques or devices evaluation (27,32,33), and, most importantly; (V) RLN injury models and prevention strategies (13,15-17,21-23,26-29,34-36). Lessons learned from these animal models not only improve understanding of current IONM technology, but also guide future research in the appropriate surgical strategies for preventing RLN injury during thyroid and parathyroid surgery. The real strength of animal modeling is in models of RLN injury which occur rarely in humans and can’t be accurately quantified in the human.
Safety considerations of electrical stimulation
In addition to RLN stimulation, vagus nerve (VN) stimulation is a recommended standard procedure during IONM to ensure that neural testing is not performed distal to the site of RLN injury (4,11). Thus, IONM requires repeated direct electrical stimulation of the RLN and the VN throughout the operation. However, there are safety concerns about the repetitive RLN and VN electrical stimulation.
Wu et al. (18) used a prospective porcine model to investigate the optimal intensity and safety of electrical VN and RLN stimulation during IONM. They found no untoward electrophysiological or cardiopulmonary effects after continuous pulsatile VN and RLN stimulations (3 mA, pulse width 100 µs, frequency 4 Hz, and duration 10 minutes). They also reported that 1 mA is a stable and safe intensity for evoking maximal EMG and suggested that regular stimulation with 1mA during IONM is the optimal intensity in terms of minimizing nerve damage risk and minimizing false-positive shunt stimuli. In Schneider et al. (27), a pig study to confirm the safety and feasibility of a new vagal anchor electrode for continuous IONM (C-IONM) revealed no vagal side effects during or after continuous VN stimulation (1.0 mA, pulse width 200 µs, frequency 3 Hz, mean duration 280 minutes). Pathologic evaluation of the VN biopsy specimen also showed that the nerve was intact with only slight epineurial edema. For surgeons, these animal studies are extremely valuable for reducing safety concerns about repetitive nerve stimulation and facilitating the formulation of valid guidelines for electrical stimulation during IONM.
Evoked L-EMG response characteristics under different stimulation/ recording conditions
As in IONM of a facial nerve during skull base, temporal, and parotid surgery, IONM of the VN/RLN, and EBSLN in thyroid surgery requires a stimulation electrode to depolarize the nerve and a recording electrode to record the evoked EMG response (4,5). Initially, IONM recordings were obtained by laryngoscopic or transcutaneous placement of needle electrodes into the vocal folds. However, the difficulty of inserting the needle electrode accurately and safely led to a search for alternative means such as placing surface electrodes in the postcricoid area or attached to an endotracheal tube (ETT) for direct contact with the vocal cord. In the past several decades, various stimulation techniques for IONM of the facial nerve have been described by Møller and Jannetta (constant-voltage stimulation) (45), Prass and Lüders (flush-tip insulation) (46), and Kartush et al. (bipolar and insulated stimulus dissectors) (47-50) and are still currently used for IONM in thyroid surgery.
In animal studies, researchers and surgeons can repeatedly stimulate or intentionally injure laryngeal nerves to compare the relative strengths or shortcomings of different stimulation or recording techniques and different IONM devices. For example, the MEEI group (16) used a canine RLN injury model to compare different recording electrode configurations (ETT surface electrodes/bipolar needle electrodes) and different methods of RLN stimulation (transesophageal/percutaneous/direct open stimulation). They confirmed that a neural integrity monitoring (NIM) system can detect typical patterns of nerve injury in intraoperative L-EMG. Additionally, IONM can be used for quantitative measurements of amplitude, latency, and wave duration in healthy and injured canine RLNs.
Wu et al. (24) used an experimental porcine model to compare L-EMG characteristics evoked by stimulation of the EBSLN/RLN/VN with ten different stimulation monopolar/bipolar probes and dissectors. The relative strengths and shortcomings of the different electrodes were also compared. They confirmed that, for all stimulation probes/dissectors, a 1-mA stimulus could evoke typical EMG waveforms from the EBSLN/VN/RLN. Notably, the stimulus-response curve showed that EMG amplitude increased as the stimulation current increased. To elicit the maximum EMG, monopolar probes and stimulation dissectors required less than 1mA whereas bipolar probes required a higher current. All stimulation electrodes recorded lower evoked EMG amplitudes (with no change in latency) when the nerve was stimulated with overlying fascia or when the distance between the probe/dissector and the nerve was increased. Improved understanding of distinguishing EMG characteristics of EBSLN/VN/RLN and normative data for different stimulation devices, different recording devices, and different conditions serves to optimize the efficacy of IONM performed by thyroid surgeons, but would also improve their ability to predict pathologic neural states.
Anesthetic management and EMG tube issues
For successful IONM of RLN, the anesthesiologist has essential roles in application of the neuromuscular blocking agent (NMBA) and in placement of the EMG tube (4). The use of NMBA is considered standard practice for safe and optimal ETT in general anesthesia. Neuromuscular blockade is needed to reduce airway trauma during tracheal intubation and to optimize mechanical ventilation (51). However, NMBA use can diminish EMG signals and interfere with intraoperative interpretation of IONM results (52-54). Lu and the KMU group (19,20) designed and performed a series of porcine studies to investigate how NMBAs affect IONM. Specifically, they compared laryngeal muscle recovery profiles in animals treated with depolarizing NMBAs (e.g., succinylcholine) and animals treated with nondepolarizing NMBAs (e.g., rocuronium) and concluded that 1 ED95 of rocuronium (0.3 mg/kg) is the optimal dose for IONM (53). Recently, they used the same porcine model to investigate the feasibility of a protocol for increasing the speed of recovery from NMBA: a standard dose of 2 ED95 rocuronium (0.6 mg/kg) combined with sugammadex. Their initial animal model and subsequent clinical study showed that sugammadex 2 mg/kg rapid and effectively restores neuromuscular function suppressed by rocuronium at the beginning of thyroid surgery (25).
In thyroid surgery, IONM of the laryngeal nerve is almost always performed by attaching a recording electrode to ETT-based surface electrodes. Therefore, an ETT that is rotated or is too deep, too shallow, or too small to contact the vocal folds can cause false negatives and other errors that can increase the risk of RLN injury (55,56). Kim and the Korean Intraoperative Neural Monitoring Society (KINMoS) (30) used a porcine model to investigate how a change in the position of the ETT can affect the EMG response. Their animal experiments revealed that rotation and depth of the ETT were significantly associated with EMG amplitude but not with EMG latency. Therefore, they proposed the concept of using “combined events” to increase reliability in tracking neural injury, i.e., using both amplitude and latency (14) to differentiate amplitude changes that were not associated with ETT malpositioning.
New techniques and devices
The number of newly developed endoscopic procedures for neck surgery has increased in the past decade. In 2005, Grunebaum et al. (32) used a porcine model to demonstrate that RLN monitoring and stimulation can be simultaneously and routinely performed during endoscopic neck surgery without major complications. They contended that RLN identification and monitoring should be considered an essential component of endoscopic neck surgery in humans. In 2009, Witzel et al. (33) used a porcine model to demonstrate the feasibility of using IONM in transoral thyroid resection.
In continuous IONM (CIONM), various stimulation electrode designs developed in recent years now enable seamless monitoring of functional integrity throughout the course of the nerve. Schneider et al. (27) used a pig model to test the feasibility of a new vagal anchor electrode for CIONM before its use in an ongoing clinical trial.
RLN injury models and prevention strategies
Elucidating the mechanisms of RLN injury not only enables further refinement of surgical techniques, but may also reduce RLN palsy rates (9-13). Researchers and surgeons can induce RLN injuries of varying severity and with varying causal mechanisms. Real-time EMG evolutions can then be recorded and correlated with vocal cord function outcome and histopathology results. Therefore, combining IONM technology with animal models provides a valuable tool for studying electrophysiology, severity, and recovery in various RLN injuries (Figure 2).
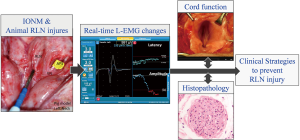
Many animal studies have already investigated the use of IONM for various RLN injuries, including traction (13,21,27,34-36), clamping and crush (13,15-17,28), thermal or cautery (13,21-23,26,27), and transection (15,16,21,29) injuries. Traction injury is an important line of research in the literature on animal IONM model. This injury commonly occurs when the thyroid lobe is mobilized and retracted during thyroidectomy. During surgical manipulation, the RLN can be over-stretched and compressed on the trachea by a dense fibrous band or a crossing artery (especially within the Berry’s ligament region) (9). Therefore, several animal studies have applied a small sustained compressive or tensile (13,21,27,34-36) stress on the RLN to simulate the clinical scenario of RLN traction injury during thyroid surgery.
Wu et al. (21) use their well-established porcine model of CIONM performed via automated periodic VN stimulation to study the EMG signal evolution during and after acute RLN traction. Detailed analyses of real-time EMG changes during RLN traction revealed a progressive amplitude decrease combined with a latency increase (the so-called “combined event”). Notably, EMG signals gradually recovered completely or at least partially after release of traction. The EMG recovery was almost complete when traction stress was relieved by 50% amplitude reduction, but the recovery was worse if the traction was relieved after loss of signal (LOS) or after prolonged and repeated application of traction to the nerve.
Several other studies have used animal CIONM models to investigate L-EMG signal changes in RLNs under traction stress. Schneider et al. (27) showed that CIONM by VN stimulation is technically feasible for predicting imminent nerve failure. Lee et al. (34) reported that traction injury of swine RLNs causes LOS at a power of 2.83 MPa. Brauckhoff et al. (35) reported that latency increase may be the first warning of RLN stretch injury during CIONM and proposed that a 50% amplitude loss can be taken as an appropriate alert limit. Lamadé et al. (36) showed that certain RLNs were 4.3 times more vulnerable to tensile force compared to the contralateral nerve. Thus, the right and the left nerves cannot be assumed to have equal sensitivity to trauma. The findings of these animal studies can help thyroid surgeons to interpret adverse EMG signals that indicate impending RLN traction injury and to use the IONM as a risk minimization tool for postoperative RLN paralysis.
Another common mechanism of intraoperative RLN injury is thermal injury, which often results from thermal spread of electrocautery and various energy-based devices (EBDs) used for hemostasis or dissection near the RLN. Lin et al. (22) used a porcine CIONM model to demonstrate that the critical temperature at which RLN thermal injury occurs is approximately 60 °C. They concluded that using CIONM to detect early stages of acute thermal stress can help to avoid further severe or repeated injury to the RLN caused by EBDs.
Since EBDs are widely used for hemostasis and dissection, their safety must be carefully evaluated, especially their effects on the RLN. In a porcine CIONM model used to evaluate the safety parameters of the Harmonic Focus (HF; Ethicon, Cincinnati, OH, USA), Wu et al. (23) found that the HF can cause unexpected iatrogenic RLN injury and recommended that its use should be standardized. The same porcine CIONM model has also been used to improve the safety of several other EBDs such as THUNDERBEAT (TB; Olympus Co Inc., Tokyo, Japan) (37) and the LigaSureTM Small Jaw (LSJ; Medtronic, Minneapolis, MN, USA) (26). The findings of these animal studies have immediate clinical applications in establishing efficient and safe strategies for using EBDs in thyroid surgery.
The main limitation of a clinical study of RLN injury is the limited availability of specimens for histopathology analysis. A clear understanding of the mechanisms and expected outcomes of RLN injuries is important not only for prognosis, but also for making correct intraoperative decisions about the extent and timing of contralateral thyroidectomy and the need for nerve repair. In Dionigi et al. (13), an electrophysiologic and histopathologic analysis of RLN injuries experimentally induced in a porcine CIONM model revealed that lesions associated with traction and mechanical injury only showed distortion of the outer nerve structure whereas lesions associated with thermal injury showed severe damage in the inner endoneurium. They calculated correlations with the clinical data for 281 RLN injuries from 6,093 at-risk nerves and defined, classified, and ranked the RLN injuries by severity. Clinicians can use this valuable combination of clinical data and data from animal studies to evaluate the severity of RLN injuries, formulate intra- and post-operative treatment, and predict recovery.
Conclusions
The IONM technology is an adjunct, not a replacement for anatomical knowledge and surgical skill. Additionally, data from animal studies may not be totally applicable to humans. Nevertheless, animal models are essential for improved understanding of IONM technology and the electrophysiology of RLN injury; data obtained from animal studies can be used to improve the efficacy of clinical techniques and strategies for preventing RLN injury during thyroid surgery.
Currently, both conventional intermittent IONM and CIONM systems have technical and interpretive limitations that can cause false-positive and false-negative errors. Therefore, future animal and clinical studies must continue to address these issues and develop innovative applications until IONM is established as a safe and easily performed standard procedure in thyroid surgery.
Acknowledgements
Funding: This study was supported by grants from the Ministry of Science and Technology (MOST 105-2314-B-037-010) and the Kaohsiung Medical University (KMU-TP105E; KMUH104-4R36), Taiwan.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Randolph GW. editor. The Recurrent and Superior Laryngeal Nerves. Cham (ZG): Springer International Publishing, 2016.
- Randolph GW. Surgery of the Thyroid and Parathyroid Glands. Philadelphia, PA: Saunders, 2013.
- Dralle H, Lorenz K, Machens A. Verdicts on malpractice claims after thyroid surgery: emerging trends and future directions. Head Neck 2012;34:1591-6. [Crossref] [PubMed]
- Randolph GW, Dralle H, International Intraoperative Monitoring Study Group, et al. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope 2011;121 Suppl 1:S1-16. [Crossref] [PubMed]
- Barczyński M, Randolph GW, Cernea CR, et al. External branch of the superior laryngeal nerve monitoring during thyroid and parathyroid surgery: International Neural Monitoring Study Group standards guideline statement. Laryngoscope 2013;123 Suppl 4:S1-14. [Crossref] [PubMed]
- Chiang FY, Lu IC, Chen HC, et al. Anatomical variations of recurrent laryngeal nerve during thyroid surgery: how to identify and handle the variations with intraoperative neuromonitoring. Kaohsiung J Med Sci 2010;26:575-83. [Crossref] [PubMed]
- Chiang FY, Lu IC, Tsai CJ, et al. Detecting and identifying nonrecurrent laryngeal nerve with the application of intraoperative neuromonitoring during thyroid and parathyroid operation. Am J Otolaryngol 2012;33:1-5. [Crossref] [PubMed]
- Kamani D, Potenza AS, Cernea CR, et al. The nonrecurrent laryngeal nerve: anatomic and electrophysiologic algorithm for reliable identification. Laryngoscope 2015;125:503-8. [Crossref] [PubMed]
- Chiang FY, Lu IC, Kuo WR, et al. The mechanism of recurrent laryngeal nerve injury during thyroid surgery--the application of intraoperative neuromonitoring. Surgery 2008;143:743-9. [Crossref] [PubMed]
- Snyder SK, Lairmore TC, Hendricks JC, et al. Elucidating mechanisms of recurrent laryngeal nerve injury during thyroidectomy and parathyroidectomy. J Am Coll Surg 2008;206:123-30. [Crossref] [PubMed]
- Chiang FY, Lee KW, Chen HC, et al. Standardization of intraoperative neuromonitoring of recurrent laryngeal nerve in thyroid operation. World J Surg 2010;34:223-9. [Crossref] [PubMed]
- Dionigi G, Alesina PF, Barczynski M, et al. Recurrent laryngeal nerve injury in video-assisted thyroidectomy: lessons learned from neuromonitoring. Surg Endosc 2012;26:2601-8. [Crossref] [PubMed]
- Dionigi G, Wu CW, Kim HY, et al. Severity of Recurrent Laryngeal Nerve Injuries in Thyroid Surgery. World J Surg 2016;40:1373-81. [Crossref] [PubMed]
- Phelan E, Schneider R, Lorenz K, et al. Continuous vagal IONM prevents recurrent laryngeal nerve paralysis by revealing initial EMG changes of impending neuropraxic injury: a prospective, multicenter study. Laryngoscope 2014;124:1498-505. [Crossref] [PubMed]
- Scott AR, Chong PS, Brigger MT, et al. Serial electromyography of the thyroarytenoid muscles using the NIM-response system in a canine model of vocal fold paralysis. Ann Otol Rhinol Laryngol 2009;118:56-66. [Crossref] [PubMed]
- Scott AR, Chong PS, Hartnick CJ, et al. Spontaneous and evoked laryngeal electromyography of the thyroarytenoid muscles: a canine model for intraoperative recurrent laryngeal nerve monitoring. Ann Otol Rhinol Laryngol 2010;119:54-63. [Crossref] [PubMed]
- Puram SV, Chow H, Wu CW, et al. Vocal cord paralysis predicted by neural monitoring electrophysiologic changes with recurrent laryngeal nerve compressive neuropraxic injury in a canine model. Head Neck 2016;38 Suppl 1:E1341-50. [Crossref] [PubMed]
- Wu CW, Lu IC, Randolph GW, et al. Investigation of optimal intensity and safety of electrical nerve stimulation during intraoperative neuromonitoring of the recurrent laryngeal nerve: a prospective porcine model. Head Neck 2010;32:1295-301. [Crossref] [PubMed]
- Lu IC, Wang HM, Kuo YW, et al. Electromyographic study of differential sensitivity to succinylcholine of the diaphragm, laryngeal and somatic muscles: a swine model. Kaohsiung J Med Sci 2010;26:640-6. [Crossref] [PubMed]
- Lu IC, Chang PY, Hsu HT, et al. A comparison between succinylcholine and rocuronium on the recovery profile of the laryngeal muscles during intraoperative neuromonitoring of the recurrent laryngeal nerve: a prospective porcine model. Kaohsiung J Med Sci 2013;29:484-7. [Crossref] [PubMed]
- Wu CW, Dionigi G, Sun H, et al. Intraoperative neuromonitoring for the early detection and prevention of RLN traction injury in thyroid surgery: a porcine model. Surgery 2014;155:329-39. [Crossref] [PubMed]
- Lin YC, Dionigi G, Randolph GW, et al. Electrophysiologic monitoring correlates of recurrent laryngeal nerve heat thermal injury in a porcine model. Laryngoscope 2015;125:E283-90. [Crossref] [PubMed]
- Wu CW, Chai YJ, Dionigi G, et al. Recurrent laryngeal nerve safety parameters of the Harmonic Focus during thyroid surgery: Porcine model using continuous monitoring. Laryngoscope 2015;125:2838-45. [Crossref] [PubMed]
- Wu CW, Liu X, Barczyński M, et al. Optimal stimulation during monitored thyroid surgery: EMG response characteristics in a porcine model. Laryngoscope 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Lu IC, Wu CW, Chang PY, et al. Reversal of rocuronium-induced neuromuscular blockade by sugammadex allows for optimization of neural monitoring of the recurrent laryngeal nerve. Laryngoscope 2016;126:1014-9. [Crossref] [PubMed]
- Dionigi G, Chiang FY, Kim HY, et al. Safety of LigaSure in recurrent laryngeal nerve dissection-porcine model using continuous monitoring. Laryngoscope 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Schneider R, Przybyl J, Pliquett U, et al. A new vagal anchor electrode for real-time monitoring of the recurrent laryngeal nerve. Am J Surg 2010;199:507-14. [Crossref] [PubMed]
- Moskalenko V, Hüller M, Gasser M, et al. Investigation of the regeneration potential of the recurrent laryngeal nerve (RLN) after compression injury, using neuromonitoring. Langenbecks Arch Surg 2009;394:469-74. [Crossref] [PubMed]
- Birkholz T, Irouschek A, Labahn D, et al. Electromyographic response persists after peripheral transection: endorsement of current concepts in recurrent laryngeal nerve monitoring in a porcine model. Langenbecks Arch Surg 2010;395:121-5. [Crossref] [PubMed]
- Kim HY, Tufano RP, Randolph G, et al. Impact of positional changes in neural monitoring endotracheal tube on amplitude and latency of electromyographic response in monitored thyroid surgery: Results from the Porcine Experiment. Head Neck 2016;38 Suppl 1:E1004-8. [Crossref] [PubMed]
- Shi Y, Hou V, Tucker A, et al. Changes of extremity and laryngeal muscle electromyographic amplitudes after intravenous administration of vecuronium. Laryngoscope 2008;118:2156-60. [Crossref] [PubMed]
- Grunebaum LD, Rosen D, Krein HD, et al. Nerve monitoring and stimulation during endoscopic neck surgery in the pig. Laryngoscope 2005;115:712-6. [Crossref] [PubMed]
- Witzel K, Benhidjeb T. Monitoring of the recurrent laryngeal nerve in totally endoscopic thyroid surgery. Eur Surg Res 2009;43:72-6. [Crossref] [PubMed]
- Lee HY, Cho YG, You JY, et al. Traction injury of the recurrent laryngeal nerve: Results of continuous intraoperative neuromonitoring in a swine model. Head Neck 2016;38:582-8. [Crossref] [PubMed]
- Brauckhoff K, Aas T, Biermann M, et al. EMG changes during continuous intraoperative neuromonitoring with sustained recurrent laryngeal nerve traction in a porcine model. Langenbecks Arch Surg 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Lamadé W, Béchu M, Lauzana E, et al. The weepy nerve-different sensitivity of left and right recurrent laryngeal nerves under tensile stress in a porcine model. Langenbecks Arch Surg 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Kwak HY, Dionigi G, Kim D, et al. Thermal injury of the recurrent laryngeal nerve by THUNDERBEAT during thyroid surgery: findings from continuous intraoperative neuromonitoring in a porcine model. J Surg Res 2016;200:177-82. [Crossref] [PubMed]
- Berke GS, Moore DM, Gerratt BR, et al. The effect of recurrent laryngeal nerve stimulation on phonation in an in vivo canine model. Laryngoscope 1989;99:977-82. [Crossref] [PubMed]
- Berke GS, Moore DM, Gerratt BR, et al. Effect of superior laryngeal nerve stimulation on phonation in an in vivo canine model. Am J Otolaryngol 1989;10:181-7. [Crossref] [PubMed]
- Kaplan EL, Salti GI, Roncella M, et al. History of the recurrent laryngeal nerve: from Galen to Lahey. World J Surg 2009;33:386-93. [Crossref] [PubMed]
- Sterpetti AV, De Toma G, De Cesare A. Recurrent laryngeal nerve: its history. World J Surg 2014;38:3138-41. [Crossref] [PubMed]
- Knight MJ, McDonald SE, Birchall MA. Intrinsic muscles and distribution of the recurrent laryngeal nerve in the pig larynx. Eur Arch Otorhinolaryngol 2005;262:281-5. [Crossref] [PubMed]
- Jiang JJ, Raviv JR, Hanson DG. Comparison of the phonation-related structures among pig, dog, white-tailed deer, and human larynges. Ann Otol Rhinol Laryngol 2001;110:1120-5. [Crossref] [PubMed]
- Tanaka H, Kobayashi E. Education and research using experimental pigs in a medical school. J Artif Organs 2006;9:136-43. [Crossref] [PubMed]
- Møller AR, Jannetta PJ. Preservation of facial function during removal of acoustic neuromas. Use of monopolar constant-voltage stimulation and EMG. J Neurosurg 1984;61:757-60. [Crossref] [PubMed]
- Prass R, Lüders H. Constant-current versus constant-voltage stimulation. J Neurosurg 1985;62:622-3. [PubMed]
- Kartush JM, Niparko JK, Bledsoe SC, et al. Intraoperative facial nerve monitoring: a comparison of stimulating electrodes. Laryngoscope 1985;95:1536-40. [Crossref] [PubMed]
- Kartush JM, Niparko JK, Graham MD, et al. Electroneurography: preoperative facial nerve assessment for tumors of the temporal bone. Otolaryngol Head Neck Surg 1987;97:257-61. [Crossref] [PubMed]
- Kartush JM. Electroneurography and intraoperative facial monitoring in contemporary neurotology. Otolaryngol Head Neck Surg 1989;101:496-503. [PubMed]
- Kartush JM, Larouere MJ, Graham MD, et al. Intraoperative cranial nerve monitoring during posterior skull base surgery. Skull Base Surg 1991;1:85-92. [Crossref] [PubMed]
- Mencke T, Echternach M, Kleinschmidt S, et al. Laryngeal morbidity and quality of tracheal intubation: a randomized controlled trial. Anesthesiology 2003;98:1049-56. [Crossref] [PubMed]
- Marusch F, Hussock J, Haring G, et al. Influence of muscle relaxation on neuromonitoring of the recurrent laryngeal nerve during thyroid surgery. Br J Anaesth 2005;94:596-600. [Crossref] [PubMed]
- Lu IC, Tsai CJ, Wu CW, et al. A comparative study between 1 and 2 effective doses of rocuronium for intraoperative neuromonitoring during thyroid surgery. Surgery 2011;149:543-8. [Crossref] [PubMed]
- Lu IC, Wu CW, Chang PY, et al. In response to Reversal of rocuronium-induced neuromuscular blockade by sugammadex allows for optimization of neural monitoring of the recurrent laryngeal nerve. Laryngoscope 2016. [Epub ahead of print]. [PubMed]
- Lu IC, Chu KS, Tsai CJ, et al. Optimal depth of NIM EMG endotracheal tube for intraoperative neuromonitoring of the recurrent laryngeal nerve during thyroidectomy. World J Surg 2008;32:1935-9. [Crossref] [PubMed]
- Tsai CJ, Tseng KY, Wang FY, et al. Electromyographic endotracheal tube placement during thyroid surgery in neuromonitoring of recurrent laryngeal nerve. Kaohsiung J Med Sci 2011;27:96-101. [Crossref] [PubMed]


