Direct-to-implant breast reconstruction
Introduction
Implant-based breast reconstruction is the most common means to restore the breast following mastectomy for breast cancer treatment or risk reduction. Many patients chose implant reconstruction secondary to the advantages of a shorter operative time, lack of donor-site morbidity, and quicker return to normal life activities. A single-stage direct-to-implant (DTI) breast reconstruction offers an ideal reconstructive choice in select patients by replacing loss of the breast at the time of the mastectomy in a single operation. In the past, DTI reconstruction was largely abandoned secondary to issues with pectoralis muscle retraction, implant malposition, and contracture. The advent of acellular dermal matrix products (ADM) offered a solution to these problems by holding the released pectoralis muscle on stretch and forming a complete pocket around the implant in the desired position (1). By off-loading stress on the inferior skin envelope, and by changing the interface of the skin envelope with the implant, it is thought that ADM-assisted reconstruction may be associated with lower contracture rates than reconstructions without ADM. A DTI procedure has obvious appeal to patient and surgeon alike, but not everyone is a candidate for single-stage reconstruction. The key to success is in patient selection, technique, and intraoperative decision-making (2).
Indications
Patient selection begins at the initial consultation. The history assesses the overall health of the patient and treatment plan, previous surgeries and co-morbidities, current medications, and smoking status. The ideal candidate for DTI reconstruction is an otherwise healthy non-smoker with a small to moderate sized breast, and who desires to be a similar breast size. If a patient wishes to be significantly larger in size, this is typically more safely done in two stages with tissue expander-implant reconstruction. Patients who have advanced disease or multiple medical co-morbidities that increase the complication risk may be better served with delayed reconstruction. Active smoking and pre-existing scars on the breast adversely affect skin perfusion and thus DTI may not be possible. Skin of the large breast may also pose challenges as it tends to become more ischemic than the skin of smaller breasts with mastectomy. Therefore, even though there is often an excess amount of skin available to use, reconstruction may need to be done in two stages or it may even need to be delayed. If the patient meets the above criteria, she is a candidate for DTI reconstruction. However, the final decision on DTI is made in the operating room based on the health and perfusion of the mastectomy skin envelope, and the surgeon should be prepared to do a tissue expander reconstruction if required.
Technique
The patient is marked preoperatively while sitting or standing. Important landmarks include the inframammary fold (IMF), the relation of the inframammary fold on one side to the other side, and the lateral borders of the breast. The optimal incision is determined with the breast oncologic surgeon. For nipple-sparing mastectomies, I find the inferolateral inframammary fold incision provides the best aesthetics while the straight lateral scar without a superior or inferior periareolar extension is the safest (3).
The patient is given a muscle relaxant to facilitate subpectoral dissection. A plane is created from lateral to medial in the fine areolar tissue beneath the pectoralis muscle to the sternal attachment of the muscle. To facilitate implant positioning, the inferior origin of the muscle is divided to the 4 o’clock or 8 o’clock position on the chest wall (1). Once the muscle is released, an acellular dermal matrix (ADM) is used as the inferior and lateral borders of the implant. In my own practice, I have the most experience with human ADM (Alloderm, Lifecell).
The ADM is sewn to the IMF inferiorly if intact or to the chest wall to create the desired IMF position. Care is taken to allow some horizontal laxity medially to accommodate the implant. Laterally, the ADM is sewn to the chest wall to create the lateral border of the breast pocket. If the ADM size is insufficient for the breast base diameter, a serratus flap may be raised laterally to gain length. A sizer is placed into the pocket and sewn into place. The skin is temporarily stapled shut and the patient is sat upright to assess pocket size and dimensions. Increasing volumes are added to the sizer while the skin is observed for signs of ischemia to help determine implant volume. The final implant is chosen based on the diameter of the breast pocket and the volume that did not induce significant ischemia. The pocket is closed over the implant. Two closed suction drains are placed with one inside the pocket along the inframammary fold (IMF) and the other outside the pocket in the axillary region. The mastectomy skin is trimmed to freshen the edges and closed in two layers. Incisions are dressed with a surgical glue (Dermabond, Ethicon) and a clear semipermeable dressing (Tegaderm, 3M) over the incision. I currently use a chlorhexidine impregnated sponge (Biopatch, Ethicon) around the drains. The implants are stabilized using microfoam tape at the lateral and inferior borders and a loose-fitting surgical bra is placed prior to discharge from the hospital in 1-2 days.
The patient is followed weekly until the drains are removed. Criteria for drain removal includes output less than 30 cc for a 24 hour time period. Activity is limited for the first six weeks to facilitate wound healing and minimize chances of implant malposition.
Outcomes
Our published institutional experience at Massachusetts General Hospital shows favorable outcomes in ADM-assisted DTI reconstruction with low total complication rates and an explant rate of 1.5% (2). There is a learning curve with the technique of DTI reconstruction that is primarily related to the ability of surgeons to determine the volume of implant the skin will be able to tolerate. If the limits of perfusion are surpassed, skin necrosis ensues. Clinical experience with the technique and in working with the oncologic surgeons yields fewer complications. Novel techniques quantifying skin perfusion (Indocyanine green perfusion imaging, laser Doppler) have the potential to shorten the learning curve for surgeons who are just starting to perform DTI reconstruction or who do so infrequently.
Although there are a number of reports associating ADM with an increased risk of infections and complications, there are also numerous studies showing no increase in complication rates, including our own paper (1,3-12). The reason for the discrepancy may reflect the learning curve in using a new product and technique. It is very important to drain the spaces adequately to prevent seroma and to limit excessive stress on the skin envelope to help prevent skin necrosis.
Patient satisfaction with DTI reconstruction is high and similar to two-stage tissue expander-implant reconstruction (unpublished data) (Figure 1).
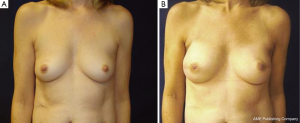
The costs associated with ADM are a frequent topic of discussion, and cost alone may be prohibitive to the availability of ADM is select regions and countries. We have shown that the cost of ADM is offset by doing the reconstruction in a single setting compared to the two surgeries required for tissue expander- implant reconstruction (2). The availability of ADM may also be limited in certain regions secondary to restrictions on the use of human or animal products. As novel matrix materials are generated and tested, their usage may become more universal.
Conclusions
Direct-to-implant breast reconstruction in properly selected patients offers excellent outcomes and patient satisfaction. The complication rate is low and improves with experience of the surgeon. If the skin envelope is determined to be healthy and sufficient at the time of the mastectomy and the patient desires a similar or smaller-sized breast, this may be the procedure of choice.
Acknowledgements
Disclosure: Amy S. Colwell M.D. is a consultant for Allergan and Lifecell. She is also a clinical investigator for a study using the AirXpander in two-stage breast reconstruction.
References
- Breuing KH, Colwell AS. Inferolateral AlloDerm hammock for implant coverage in breast reconstruction. Ann Plast Surg 2007;59:250-5.
- Colwell AS, Damjanovic B, Zahedi B, et al. Retrospective review of 331 consecutive immediate single-stage implant reconstructions with acellular dermal matrix: indications, complications, trends, and costs. Plast Reconstr Surg 2011;128:1170-8.
- Colwell AS, Gadd M, Smith BL, et al. An inferolateral approach to nipple-sparing mastectomy: optimizing mastectomy and reconstruction. Ann Plast Surg 2010;65:140-3.
- Breuing KH, Colwell AS. Immediate breast tissue expander-implant reconstruction with inferolateral AlloDerm hammock and postoperative radiation: a preliminary report. Eplasty 2009;9:e16.
- Breuing KH, Warren SM. Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg 2005;55:232-9.
- Chun YS, Verma K, Rosen H, et al. Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg 2010;125:429-36.
- Kim JY, Davila AA, Persing S, et al. A meta-analysis of human acellular dermis and submuscular tissue expander breast reconstruction. Plast Reconstr Surg 2012;129:28-41.
- Namnoum JD. Expander/implant reconstruction with AlloDerm: recent experience. Plast Reconstr Surg 2009;124:387-94.
- Peled AW, Foster RD, Garwood ER, et al. The effects of acellular dermal matrix in expander-implant breast reconstruction after total skin-sparing mastectomy: results of a prospective practice improvement study. Plast Reconstr Surg 2012;129:901e-908e.
- Salzberg CA, Ashikari AY, Koch RM, et al. An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (AlloDerm). Plast Reconstr Surg 2011;127:514-24.
- Weichman KE, Wilson SC, Weinstein AL, et al. The use of acellular dermal matrix in immediate two-stage tissue expander breast reconstruction. Plast Reconstr Surg 2012;129:1049-58.
- Zienowicz RJ, Karacaoglu E. Implant-based breast reconstruction with allograft. Plast Reconstr Surg 2007;120:373-81.


