Does neoadjuvant chemotherapy affect morbidity, mortality, reoperations, or readmissions in patients undergoing lumpectomy or mastectomy for breast cancer?
Introduction
The use of neoadjuvant chemotherapy (NAC) in breast cancer patients is increasing (1,2). Although the hope of NAC to improve cancer specific survival has not yet been realized, there is good evidence that NAC benefits patients by increasing their chance of successful breast-conserving therapy (BCT) (1,2). Patients previously excluded from the option of BCT, such as those with multi-focal tumors or tumors larger than 5 cm, increasingly undergo lumpectomy after NAC (3). In recent reports, their cancer outcomes were equivalent to those who underwent mastectomy (4,5). Patient-centered drivers of NAC also exist. If NAC is undertaken, there is time during which the patient can undergo genetic testing and plastic surgical consultation. Both aid decision making in those patients initially uncertain about their preference between mastectomy and lumpectomy for their treatment. In addition, premenopausal patients interested in future pregnancy have time for fertility counseling. For all these reasons, the number of patients receiving NAC in the future is expected to rise.
In contrast to the well-established benefit of NAC for breast preservation, information on the influence of NAC on postoperative surgical outcomes after breast surgery is incomplete. Utilizing the statistical power of national databases, only a few studies have reported postoperative outcomes after receipt of NAC in patients with breast cancer (6-9). Due to the smaller number of patient encounters, single-institution reports of the effect of NAC on postoperative complications after breast surgery are more limited in their ability to distinguish differences in complications attributed to NAC (10-12). The association of NAC with morbidity and mortality (M&M) for organ sites other than breast has been reported many times (13-26), but these reports may not be relevant for breast patients. For example, patients undergoing breast surgery have lower overall M&M but higher reoperation rates compared with most other general surgical oncology operations (27-29). In addition, there are differences in the types of chemotherapy and targeted agents delivered to patients dependent on cancer type, further decreasing the relevance of NAC studies of other organs. To date, when all organ sites are considered, the findings of the influence of NAC on postoperative outcomes are mixed (6-26). Given the uncertain effects of NAC on surgical outcomes, the primary aim of this study was to utilize the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database to characterize the impact of NAC on postoperative complications after lumpectomy and mastectomy, the two most common breast cancer operations.
Methods
We obtained Gundersen Clinic, Ltd. Human Subjects Committee/Institutional Review Board exemption for this study. Use of the de-identified NSQIP Participant Use Data File (PUF) is Health Insurance Portability and Accountability Act (HIPAA)-compliant (30).
The NSQIP database was used to determine associations between the predictor variable receipt of NAC within 30 days of surgery and the primary study outcomes of serious morbidity and/or mortality within 30 days of surgery, reoperation rates within 30 days of surgery, readmission rates within 30 days of surgery, and duration of operative time-skin to skin (8,31-35). The composite (summative) performance measure of serious morbidity and/or mortality was used instead of individual morbidity measures, owing to the low number of these individual events in breast patients (27,28,33). This composite measure is endorsed by the National Quality Forum (35). It has also been validated and used extensively by other investigators and has been termed the “primary outcome measure of NSQIP” (33). Composite measures better reflect hospital quality than simple rates of risk-adjusted morbidity. Serious morbidity indicates one or more of the following events occurred: cardiac arrest, myocardial infarction, pneumonia, progressive renal insufficiency, acute renal failure, venous thromboembolism, deep incisional surgical site infection (SSI), organ space SSI, sepsis, septic shock, unplanned intubation, urinary tract infection, wound disruption, or reoperation. Readmission, first appearing as a NSQIP outcome in 2011, has not yet been incorporated into the composite M&M measure.
Inclusion criteria included those with the International Classification of Disease, 9th Revision (ICD-9) diagnosis codes for lumpectomy for cancer (174.0–174.9), lumpectomy-other (217, 611.72, 610.0–610.9, 611.0–611.79, 611.8–611.9, 793.8–793.89), and mastectomy (174.0–174.9, 233.0), and the common procedural term (CPT) codes for patients undergoing breast lumpectomy, mastectomy of any type, sentinel node biopsy, or axillary dissection (19160, 19162, 19180, 19182, 19240, 19301, 19302, 19303, 19304, and 19307). Any combination of these CPT codes was acceptable. By design, NSQIP limits the number of smaller outpatient operations, such as lumpectomy, to a maximum of three cases during each of the 46 standard 8-day NSQIP sampling cycles in a year (36). This limit minimizes bias in comparisons of institutions for overall general surgical M&M. Patients were excluded if they had a concurrent operation the CPT code of which was not one of the codes listed above. Thus, patients undergoing breast reconstruction were excluded. Patients were also excluded if they were male, pregnant, had disseminated cancer, had radiotherapy for malignancy in the last 90 days, underwent an emergency operation, or if the total operation time was less than 15 minutes. Patients with missing values for our predictor variable, confounding variables, or our outcomes were also excluded.
The statistical power for detecting the effect of NAC on M&M was investigated using simulation a priori. The simulated power analysis used sample sizes and the overall M&M rate from the observed data from 2005 to 2010, from 2011 to 2012, and the combined data from 2005 to 2012. The power for detecting a NAC effect was simulated for a wide range of odds ratios (ORs) using a logistic model with the NAC predictor variable and one binary confounding variable. For each OR value, M&M data were simulated using the binomial distribution, and a logistic model was fit to these simulated data 1,000 times.
Separate analyses were performed for years 2005 to 2010 and 2011 to 2012, owing to changes in NSQIP definitions beginning in 2011. Present at time of surgery (PATOS) variables were introduced in 2011. PATOS variables are used to remove postoperative morbidity events when they were present preoperatively. There was also a change in the reoperation variable beginning in 2011. Before 2011, NSQIP reported a variable for a reoperation for any reason, including reoperation for a close or positive surgical margin. In 2011, NSQIP introduced a different reoperation variable that captured unplanned reoperations related to the original or concurrent procedure but excluded reoperations for margins. The final change that began in 2011 was that reporting for the data field NAC within 30 days of surgery was changed from mandatory to optional for patients with cancer.
All analyses were performed with SAS 9.3 software (SAS Institute, Cary, NC, USA). Patient characteristics and comorbidities (confounding covariates) and differences between the two study groups stratified by NAC within 30 days of surgery were compared by χ2 or Fisher’s exact test (two-tailed P values with significance <0.05). The unadjusted univariable analysis of primary study outcomes and mortality stratified by the variable NAC within 30 days of surgery were also compared by χ2 test with significance established at a P value <0.05. An unadjusted univariable analysis of the individual morbidity outcomes that comprise the composite serious M&M for lumpectomy patients was also performed [Fisher’s exact test P values (two-tailed P values with significance <0.05)]. Multiple logistic regression models were then developed to analyze the association between NAC within 30 days of surgery and our primary study outcomes. We adjusted for clinically and statistically relevant confounders. The significant predictors in the univariable analysis (P<0.05) were included in the multivariable models.
The confounding covariates used for our logistic regression models included patient age, operation year, American Society of Anesthesiologists (ASA) class, body mass index (BMI), diabetes mellitus (with oral agents or insulin), steroid use for chronic condition, hypertension requiring medication, bleeding disorder, smoking status, preoperative functional status, congestive heart failure, dyspnea, chronic obstructive pulmonary disease (COPD), preoperative serum albumin <3.0 g/dL, renal failure or dialysis, systemic inflammatory response (SIRS) syndrome, sepsis/septic shock, and wound classification. These covariates have been used in prior NSQIP investigations for both breast and non-breast general surgical operations (8,33). Due to recent reports using the NSQIP database that identified differences in M&M stratified by procedure type (lumpectomy versus mastectomy), we enhanced our regression model by including procedure type as a covariate (9,37,38). Two covariates used by others for risk adjustment for general surgical operations, ventilator dependence and ascites, were excluded from our analysis because we found them to be exceptionally rare conditions in patients undergoing breast cancer operations.
A secondary study outcome, the effect of NAC on duration of time for the operation (NSQIP skin-to-skin data field), was also analyzed. The estimated effects of NAC and operative procedure type on mean operative times for all patients during 2005–2012 were calculated by using a generalized linear model with log link and gamma distribution. This model choice parallels the methods of a recently published study using the NSQIP database (34).
A comprehensive but nonsystematic review of the NSQIP literature for breast surgery was performed with the search terms of “neoadjuvant and NSQIP” and “NSQIP and chemotherapy”. A secondary search was performed of the relevant references provided in the above publications. See Table 1.
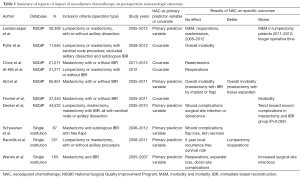
Full table
Results
From 2005–2012, there were 37,902 patients meeting inclusion criteria (Figure 1). After excluding all cases with missing values for any response, predictor, or confounding variable, there were 30,309 evaluable patients.
Based on the power analysis, the logistic model using the combined 2005–2012 sample size has at least 80% power for detecting an OR of 0.75 (or reciprocal 1.33) or more extreme when contrasting the odds of M&M for those who did not receive NAC to those who received it. Based on the 2005–2010 sample size, there is at least 80% power for detecting an OR of 0.73 (or reciprocal 1.37) or more extreme. The 2011–2012 sample size has at least 80% power for detecting an OR of 0.54 (or reciprocal 1.85).
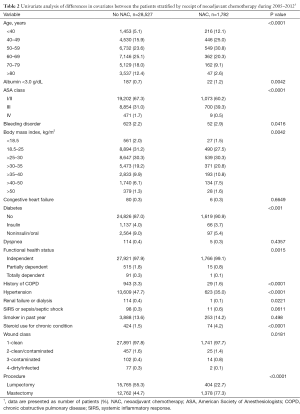
There were differences in nearly all covariates between the patient groups stratified by NAC within 30 days of surgery (Table 2). Patients receiving NAC more often underwent mastectomy and were younger and had more steroid use, ASA class III wounds, albumin <3.0 g/dL, BMI >25 kg/m2, bleeding disorders, and independent functional status. Patients not receiving NAC had more COPD, hypertension, renal failure, and diabetes. The groups were similar with regard to smoking status, dyspnea, sepsis, and congestive heart failure.
Full table
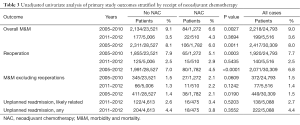
The univariate analysis of our primary study outcomes stratified by NAC within 30 days of surgery is shown in Table 3. With this unadjusted analysis, inclusive of all study years [2005–2012], our primary study outcomes for M&M, M&M without reoperations, and reoperations were associated with receipt of NAC.
Full table
The risk-adjusted multiple logistic regression analysis of our study outcomes stratified by NAC within 30 days of surgery are provided in Table 4. For the years 2005–2012, NAC was not associated with the composite measure of postoperative M&M for all procedure types [P=0.376; OR 0.911; 95% confidence interval (CI), 0.740–1.121], mastectomy or lumpectomy reoperations (P=0.293; OR 0.881; 95% CI, 0.696–1.116), mastectomy reoperations (P=0.153; OR 0.792; 95% CI, 0.575–1.090), or lumpectomy reoperations (P=0.947; OR 1.012; 95% CI, 0.715–1.432). For the years 2011–2012, NAC was not significantly associated with readmissions related to index procedure (P=0.528; OR 1.258; 95% CI, 0.710–2.229) or with readmissions related or not to index procedure (P=0.412; OR 0.805; 95% CI, 0.479–1.352). Twenty-four (0.08%) of 30,309 patients died within 30 days of surgery: 2 (0.11%) of 1,782 with NAC and 22 (0.08%) of 28,527 without NAC (P=0.609).
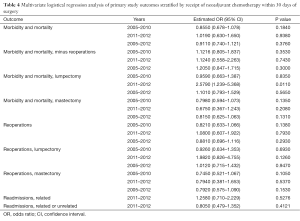
Full table
After segregation into the time periods before and after NSQIP changes in definitions for reoperations, the only significant association of any outcome with NAC was for higher M&M in lumpectomy patients during 2011–2012 (P=0.011; OR 2.579; 95% CI, 1.239–5.368). A search was then undertaken to determine whether there were differences between the NAC groups for the individual events that make up this composite M&M performance measure (Table 5). Compared with patients who had not received NAC, patients who had received NAC had a higher percentage of reoperations, deep incisional SSI, organ space SSI, and urinary tract infections, but none of these differences reached statistical significance.
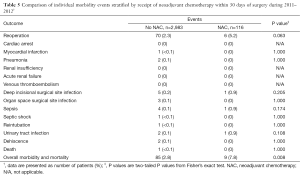
Full table
For all patients, all procedures, and all years of study, mean operative times (skin-to-skin) for mastectomy and lumpectomy patients were 115 and 61 minutes, respectively. Mean operative times in patients who received and did not receive NAC for all patients were 123 and 83 minutes, respectively. Using the duration of time for operation model described by Daley et al. (34), the estimated effects of no NAC compared with receipt of NAC on operation time were 0.8436, 95% CI, 0.8224–0.8653 (P value <0.0001), demonstrating longer operation times in patients receiving NAC. The geometric mean operative time in patients without NAC was about 0.8 times that with NAC.
Discussion
Excluding cutaneous cancer, breast cancer is the most common cancer in women in the United States (39). During the last decade, there is no doubt that NAC prior to breast surgery has increased in these patients (1,2). The primary driver of this change is the ability of NAC to increase BCT rates (1,2). In addition, the effectiveness of NAC to achieve a complete pathologic treatment response in selected patients generates enthusiasm for its use (40-42). As new, tumor-specific, multi-gene molecular signatures are discovered, it is also likely that novel targeted agents will be introduced into clinical trials, often in the neoadjuvant setting. Thus, it is anticipated that the proportion of all newly diagnosed breast cancer patients undergoing NAC will increase.
Key questions regarding the influence of induction chemotherapy on postoperative outcomes after breast surgery remain unanswered. Does NAC increase the rate of surgical complications? If so, what types of complications? Since nearly all newly diagnosed breast cancer patients undergo surgery, it behooves us to study the impact of NAC on postoperative outcomes. Armed with increased understanding, the shared decision making and informed consent processes between provider and patient regarding the option of lumpectomy versus mastectomy and neoadjuvant versus postoperative adjuvant systemic treatment can be improved. Furthermore, if patient subpopulations are identified that have increased specific or unique morbidities from NAC, then strategies can be developed to either identify them early or mitigate their chance of occurring.
Although the exact influence of NAC on postoperative complication rates is not fully established, Abt et al. commented that “most” surgeons believe that NAC increases morbidity (8). The results of prior investigations of NAC depend on the organ studied, procedure type, complication type, agents used, and whether radiation was delivered preoperatively (6-9,13,16-25). Findings also vary between studies of the same organ (Table 1) (6-12,37). In some reports of organ sites other than breast, NAC was associated with increased transfusions, readmissions, SSI, stroke, and mortality (13,15,21,22,24,25). In other reports, NAC was not associated with any complication (13-21,23,25). Given this variability, our primary objective was to clarify the association between NAC and non-oncologic postoperative outcomes in patients undergoing breast surgery.
The strengths of the NSQIP database to identify and compare postoperative surgical outcomes have been well described (43-45). With NSQIP, the number of patient encounters accessible for review far exceeds that of single institutional and regional databases, allowing better discriminatory power to identify even small differences between patient groups stratified by patient characteristics, comorbidities, or interventions. Prior studies of patients undergoing breast operations have demonstrated low overall M&M (27,28). The rates of complications are lower than those in patients undergoing more complex general surgical operations. The profile of postoperative complication types also differs between breast and general surgical operations. For example, reoperations for margins are more common in patients undergoing breast operations (29). These and other differences suggest that prior reports on the association between NAC and outcomes for other organ sites may not be relevant for breast. By the use of procedural codes, the NSQIP database can segregate breast from non-breast patient groups.
Prior publications using the NSQIP database for interrogation of breast outcomes that recorded NAC as a predictor variable are sparse. None have been inclusive of all operation types. Some are limited in scope—that is, restricted to a single study year or procedure or to a single outcome measure, such as reoperations. Most report on individual outcome measures, such as SSI or bleeding, rather than the primary outcome measure of NSQIP, the composite measure of M&M. Since individual measures of morbidity occur so infrequently after breast surgery, investigations of single outcome measures, even in NSQIP, may be underpowered (6). A summative measure of postoperative complications, such as used herein, would be more likely to identify differences stratified by receipt of NAC. To our knowledge, only six investigators have reported on the effects of NAC in patients undergoing breast operations using the NSQIP database (6-9,37,46), and only two of these used NAC as the primary predictor variable (Table 1). Only a single study by Abt et al. (8) used, as we did, NAC as the predictor variable and the composite M&M measure as the outcome measure.
In the current study, patients undergoing lumpectomy or mastectomy—with or without axillary surgery—had no increase in M&M, the primary postoperative outcome measure of NSQIP, except for patients undergoing lumpectomy in the years 2011–2012. After scrutiny of the individual measures that comprise the composite M&M measure, lumpectomy patients with NAC during these 2 years had higher rates of reoperations, deep incisional SSI, sepsis, and urinary tract infections, but none of these events reached statistical significance (Table 5). Since the NSQIP data field for NAC was dropped after 2012, it is no longer possible to determine whether the association between NAC and M&M still exists for lumpectomy patients.
We identified no association between NAC and reoperations or readmissions during any period of our study for either lumpectomy or mastectomy, corroborating prior reports that utilized slightly different analytic methodologies and procedure types, including reconstruction (7,10,12). Although the absolute differences were often small, we found multiple significant differences between patient characteristics (confounding variables) by receipt of NAC in the NSQIP registry (Table 2). These covariates should, therefore, be incorporated into regression models used in future investigations of the effect of NAC on postoperative outcomes. Confirming prior reports from many authors, we found the overall morbidity to be less than 10% after breast cancer operations. Combining all study years and both procedure types, the rates of composite M&M and readmissions was 8% and 4.4%, respectively. It should be noted that reoperations are included in this composite measure. In the years 2011–2012, reoperation rates were only 2.5%, but during these years NSQIP did not include reoperations for positive margins in their reoperation rate definition. Further review of reoperation rates, found in Figure 2 and Table 3, indicates that the largest contributor to the summative NSQIP composite measure of M&M proportionately is reoperations.
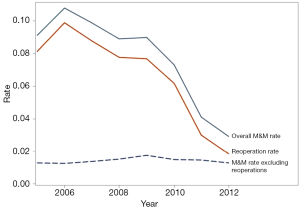
The association between NAC and duration of surgical procedure time has been sparsely reported. In separate NSQIP studies of esophageal and bladder cancer, operative times did not differ based on receipt of NAC (13,20). In contrast, Abt et al. (8) reported longer operative times in mastectomy patients receiving NAC compared with those who did not. In agreement, we identified an association between NAC and increased operative time. The reasons for this are unknown but are probably due to more mastectomy than lumpectomy patients in our patient cohort receiving NAC. The absence of cancer-specific staging data, tumor size, and breast size in the NSQIP database limits analyses of these contributors to operative time. However, given the recently reported finding that operations that are “too long” in NSQIP can be associated with postoperative M&M, further investigations into causes of long operative times are warranted (34).
The NSQIP database has many strengths (44). Within NSQIP, enough diversity by geography, hospital type, and patient demographics exists to increase the generalizability of study findings. The database is large, providing discriminatory power during comparisons of predictor variables. Patient characteristics and comorbidities that influence outcomes are known, allowing for risk adjustment, and are entered by trained abstractors, enhancing accuracy. Furthermore, a composite measure of outcome performance—serious M&M—has been developed, with standardized specifications for confounding covariates available for use in the development of regression models. Unlike prior regression models using NSQIP to study breast outcomes, our models included procedure type, owing to recent reports of increased M&M with mastectomy compared with lumpectomy (37). Another strength of our analyses was our segregation of outcomes reporting into two time periods. As previously mentioned, this was necessary because the NSQIP definition for a key outcome measure—reoperation—changed after 2010, and reoperations comprise part of NSQIP’s composite M&M measure.
The NSQIP database is not without limitations for the investigation of outcomes after breast surgery or the influence of NAC (6). Not all lumpectomies are reported. They are randomly selected, but case capture is limited to three lumpectomies per 8-day reporting cycle. In addition, some common breast-specific morbid complications are not captured at all, such as seroma and lymphedema. Furthermore, the NSQIP database does not include some confounding covariates that are known to increase reoperation rates, such as tumor size and cancer stage. The lack of these cancer-specific variables on models aiming to provide risk-adjusted peer comparisons of surgical outcomes after oncology operations has been addressed by Merkow et al. (47,48). Lastly, NSQIP does not record information on the type of chemotherapy. Safety profiles could differ by agent. If available, information on tumor characteristics and more detail about chemotherapy agents would enhance our multivariable analysis of surgical complications. The addition of just a few breast-specific variables, including tumor staging, could enhance and broaden the use of NSQIP for investigators of breast M&M, reoperations, and readmissions. Precedent for organ-specific additions to NSQIP’s database, including receipt of NAC, have recently occurred for hepatic surgeons (49).
Given the robust nature of the NSQIP database, as well as to the marked increase in use of NAC during the last decade, it is not surprising that many investigators from multiple subspecialties are using this database to determine its influence on M&M. However, there are substantial limitations to all these efforts, including the use of the NSQIP descriptor for NAC, NAC within 30 days of surgery, as a predictor variable. All prior reports, including our own, have used this descriptor as a surrogate for receipt of NAC, even though some patients who received NAC will be incorrectly classified as not having received NAC if the last chemotherapy was delivered more than 30 days before surgery. Some authors, but not all, acknowledge this limitation of the NSQIP descriptor. Prior authors have justified using the NSQIP definition for NAC as an appropriate surrogate, referencing past studies that indicate most operations do occur within 30 days of the last NAC cycle (8). This predictor variable also does not allow for analysis of the linear influence of the duration of time between NAC and operation—or whether there is an inflection point between timing of NAC and its effect on outcomes—because the date field response choices are categorical and binary (“yes” or “no”). In other words, it is not possible to determine whether there is an optimal interval in days for recovery between NAC and surgery regarding risk of postoperative complications. Lastly, NSQIP discontinued the NAC field after 2012; even before discontinuation, many values were missing. Given the aforementioned restrictions of NSQIP to include no data field for NAC after 2012, a data descriptor that can misclassify patients by receipt of NAC and many missing values, we and others have not yet proven that NAC has no association with postoperative surgical outcomes. To assume there is no association based on the current NSQIP data, all prior to 2013, could be perilous.
We conclude that from 2005–2012 NAC was not associated with an increase in the primary postoperative outcome measures of NSQIP: serious M&M, mortality, reoperations, or readmissions. However, for unknown reasons one subgroup—lumpectomy patients in 2011 and 2012—had higher M&M with NAC, and it was not possible to study later years due to discontinuation of the NAC data field after 2012. Overall, these observations are consistent with the existing literature, which generally supports the safety of NAC but also identifies occasional subgroups with increased morbidity. This persistent lack of clarity provides a compelling reason to continue to track the impact of NAC on postoperative outcomes during the next decade, a time during which use of NAC will increase and newer agents will be introduced. We recommend reinstitution of a NAC data field into the NSQIP program.
Acknowledgements
The authors thank Courtney Mumm, Shaun Fleischhacker, and Jonathan Forsythe for their assistance with regression analysis, Cathy Mikkelson Fischer, MA, ELS, for her assistance with editing and manuscript preparation, and Travis Smith, MD, and Pamela Lambert, RN, for their assistance with the NSQIP Participant Use Data File (PUF). This work was supported by Gundersen Medical Foundation and the Norma J. Vinger Center for Breast Care.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: We obtained Gundersen Clinic, Ltd. Human Subjects Committee/Institutional Review Board exemption for this study.
References
- Golshan M, Cirrincione CT, Sikov WM, et al. Impact of neoadjuvant chemotherapy in stage II-III triple negative breast cancer on eligibility for breast-conserving surgery and breast conservation rates: surgical results from CALGB 40603 (Alliance). Ann Surg 2015;262:434-9; discussion 438-9. [Crossref] [PubMed]
- Killelea BK, Yang VQ, Mougalian S, et al. Neoadjuvant chemotherapy for breast cancer increases the rate of breast conservation: results from the National Cancer Database. J Am Coll Surg 2015;220:1063-9. [Crossref] [PubMed]
- Baron P, Beitsch P, Boselli D, et al. Impact of Tumor Size on Probability of Pathologic Complete Response After Neoadjuvant Chemotherapy. Ann Surg Oncol 2016;23:1522-9. [Crossref] [PubMed]
- Bleicher RJ, Ruth K, Sigurdson ER, et al. Breast conservation versus mastectomy for patients with T3 primary tumors (>5 cm): A review of 5685 medicare patients. Cancer 2016;122:42-9. [Crossref] [PubMed]
- Ataseven B, Lederer B, Blohmer JU, et al. Impact of multifocal or multicentric disease on surgery and locoregional, distant and overall survival of 6,134 breast cancer patients treated with neoadjuvant chemotherapy. Ann Surg Oncol 2015;22:1118-27. [Crossref] [PubMed]
- Decker MR, Greenblatt DY, Havlena J, et al. Impact of neoadjuvant chemotherapy on wound complications after breast surgery. Surgery 2012;152:382-8. [Crossref] [PubMed]
- Chow I, Hanwright PJ, Hansen NM, et al. Predictors of 30-day readmission after mastectomy: A multi-institutional analysis of 21,271 patients. Breast Dis 2015;35:221-31. [Crossref] [PubMed]
- Abt NB, Flores JM, Baltodano PA, et al. Neoadjuvant chemotherapy and short-term morbidity in patients undergoing mastectomy with and without breast reconstruction. JAMA Surg 2014;149:1068-76. [Crossref] [PubMed]
- Al-Hilli Z, Thomsen KM, Habermann EB, et al. Reoperation for Complications after Lumpectomy and Mastectomy for Breast Cancer from the 2012 National Surgical Quality Improvement Program (ACS-NSQIP). Ann Surg Oncol 2015;22 Suppl 3:S459-69. [Crossref] [PubMed]
- Warren Peled A, Itakura K, Foster RD, et al. Impact of chemotherapy on postoperative complications after mastectomy and immediate breast reconstruction. Arch Surg 2010;145:880-5. [Crossref] [PubMed]
- Schaverien MV, Munnoch DA. Effect of neoadjuvant chemotherapy on outcomes of immediate free autologous breast reconstruction. Eur J Surg Oncol 2013;39:430-6. [Crossref] [PubMed]
- Karanlik H, Ozgur I, Cabioglu N, et al. Preoperative chemotherapy for T2 breast cancer is associated with improved surgical outcome. Eur J Surg Oncol 2015;41:1226-33. [Crossref] [PubMed]
- Mungo B, Molena D, Stem M, et al. Does neoadjuvant therapy for esophageal cancer increase postoperative morbidity or mortality? Dis Esophagus 2015;28:644-51. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Lv J, Cao XF, Zhu B, et al. Effect of neoadjuvant chemoradiotherapy on prognosis and surgery for esophageal carcinoma. World J Gastroenterol 2009;15:4962-8. [Crossref] [PubMed]
- Fahy BN, Aloia TA, Jones SL, et al. Chemotherapy within 30 days prior to liver resection does not increase postoperative morbidity or mortality. HPB (Oxford) 2009;11:645-55. [Crossref] [PubMed]
- Cho SW, Tzeng CW, Johnston WC, et al. Neoadjuvant radiation therapy and its impact on complications after pancreaticoduodenectomy for pancreatic cancer: analysis of the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP). HPB (Oxford) 2014;16:350-6. [Crossref] [PubMed]
- Cooper AB, Parmar AD, Riall TS, et al. Does the use of neoadjuvant therapy for pancreatic adenocarcinoma increase postoperative morbidity and mortality rates? J Gastrointest Surg 2015;19:80-6; discussion 86-7. [Crossref] [PubMed]
- Lee GC, Fong ZV, Ferrone CR, et al. High performing whipple patients: factors associated with short length of stay after open pancreaticoduodenectomy. J Gastrointest Surg 2014;18:1760-9. [Crossref] [PubMed]
- Johnson DC, Nielsen ME, Matthews J, et al. Neoadjuvant chemotherapy for bladder cancer does not increase risk of perioperative morbidity. BJU Int 2014;114:221-8. [Crossref] [PubMed]
- Tyson MD 2nd, Bryce AH, Ho TH, et al. Perioperative complications after neoadjuvant chemotherapy and radical cystectomy for bladder cancer. Can J Urol 2014;21:7259-65. [PubMed]
- Téoule P, Trojan J, Bechstein W, et al. Impact of Neoadjuvant Chemotherapy on Postoperative Morbidity after Gastrectomy for Gastric Cancer. Dig Surg 2015;32:229-37. [Crossref] [PubMed]
- Musters GD, Sloothaak DA, Roodbeen S, et al. Perineal wound healing after abdominoperineal resection for rectal cancer: a two-centre experience in the era of intensified oncological treatment. Int J Colorectal Dis 2014;29:1151-7. [Crossref] [PubMed]
- Abt NB, Bydon M, De la Garza-Ramos R, et al. Concurrent neoadjuvant chemotherapy is an independent risk factor of stroke, all-cause morbidity, and mortality in patients undergoing brain tumor resection. J Clin Neurosci 2014;21:1895-900. [Crossref] [PubMed]
- Hein PN, Lieber B, Bruce E, et al. Influence on morbidity and mortality of neoadjuvant radiation and chemotherapy among cranial malignancy patients in the postoperative setting. J Clin Neurosci 2015;22:998-1001. [Crossref] [PubMed]
- Bartlett EK, Roses RE, Meise C, et al. Preoperative radiation for retroperitoneal sarcoma is not associated with increased early postoperative morbidity. J Surg Oncol 2014;109:606-11. [Crossref] [PubMed]
- El-Tamer MB, Ward BM, Schifftner T, et al. Morbidity and mortality following breast cancer surgery in women: national benchmarks for standards of care. Ann Surg 2007;245:665-71. [Crossref] [PubMed]
- Neumayer L, Schifftner TL, Henderson WG, et al. Breast cancer surgery in Veterans Affairs and selected university medical centers: results of the patient safety in surgery study. J Am Coll Surg 2007;204:1235-41. [Crossref] [PubMed]
- Eck DL, Koonce SL, Goldberg RF, et al. Breast surgery outcomes as quality measures according to the NSQIP database. Ann Surg Oncol 2012;19:3212-7. [Crossref] [PubMed]
- American College of Surgeons. Inspiring Quality: Highest Standards, Better Outcomes. ACS NSQIP Participant Use Data File, 2016. Available online: https://www.facs.org/quality-programs/acs-nsqip/program-specifics/participant-use
- Dimick JB, Staiger DO, Hall BL, et al. Composite measures for profiling hospitals on surgical morbidity. Ann Surg 2013;257:67-72. [Crossref] [PubMed]
- Osborne NH, Nicholas LH, Ryan AM, et al. Association of hospital participation in a quality reporting program with surgical outcomes and expenditures for Medicare beneficiaries. JAMA 2015;313:496-504. [Crossref] [PubMed]
- Rajaram R, Chung JW, Cohen ME, et al. Association of the 2011 ACGME Resident Duty Hour Reform with Postoperative Patient Outcomes in Surgical Specialties. J Am Coll Surg 2015;221:748-57. [Crossref] [PubMed]
- Daley BJ, Cecil W, Clarke PC, et al. How slow is too slow? Correlation of operative time to complications: an analysis from the Tennessee Surgical Quality Collaborative. J Am Coll Surg 2015;220:550-8. [Crossref] [PubMed]
- National Quality Forum: Quality Positioning System: Measure Number 0697. Risk adjusted case mix adjusted elderly surgery outcomes measure 2012. Available online: http://www.qualityforum.org/QPS/QPSTool.aspx#qpsPageState=%7B%22TabType%22%3A1,%22TabContentType%22%3A1,%22SearchCriteriaForStandard%22%3A%7B%22TaxonomyIDs%22%3A%5B%5D,%22SelectedTypeAheadFilterOption%22%3A%7B%22ID%22%3A11,%22FilterOptionLabel%22%3A%220697%22,%22TypeOfTypeAheadFilterOption%22%3A4,%22TaxonomyId%22%3A0%7D,%22Keyword%22%3A%220697%22,%22PageSize%22%3A%2225%22,%22OrderType%22%3A3,%22OrderBy%22%3A%22ASC%22,%22PageNo%22%3A1,%22IsExactMatch%22%3Afalse,%22QueryStringType%22%3A%22%22,%22ProjectActivityId%22%3A%220%22,%22FederalProgramYear%22%3A%220%22,%22FederalFiscalYear%22%3A%220%22,%22FilterTypes%22%3A0,%22EndorsementStatus%22%3A%22%22%7D,%22SearchCriteriaForForPortfolio%22%3A%7B%22Tags%22%3A%5B%5D,%22FilterTypes%22%3A0,%22PageStartIndex%22%3A1,%22PageEndIndex%22%3A25,%22PageNumber%22%3Anull,%22PageSize%22%3A%2225%22,%22SortBy%22%3A%22Title%22,%22SortOrder%22%3A%22ASC%22,%22SearchTerm%22%3A%22%22%7D,%22ItemsToCompare%22%3A%5B%5D,%22SelectedStandardIdList%22%3A%5B%5D%7D
- American College of Surgeons. User Guide for the 2012 ACS NSQIP Participant Use Data File. Chicago, Illinois: American College of Surgeons; 2013. Available online: https://www.facs.org/~/media/files/quality%20programs/nsqip/ug12.ashx
- Pyfer B, Chatterjee A, Chen L, et al. Early Postoperative Outcomes in Breast Conservation Surgery Versus Simple Mastectomy with Implant Reconstruction: A NSQIP Analysis of 11,645 Patients. Ann Surg Oncol 2016;23:92-8. [Crossref] [PubMed]
- Chatterjee A, Pyfer B, Czerniecki B, et al. Early postoperative outcomes in lumpectomy versus simple mastectomy. J Surg Res 2015;198:143-8. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Morrow M. Parsing Pathologic Complete Response in Patients Receiving Neoadjuvant Chemotherapy for Breast Cancer. JAMA Oncol 2016;2:516-7. [Crossref] [PubMed]
- Chia SK. Neoadjuvant and Adjuvant Therapy for HER2 Positive Disease. Am Soc Clin Oncol Educ Book 2015.e41-8. [Crossref] [PubMed]
- Mougalian SS, Hernandez M, Lei X, et al. Ten-Year Outcomes of Patients With Breast Cancer With Cytologically Confirmed Axillary Lymph Node Metastases and Pathologic Complete Response After Primary Systemic Chemotherapy. JAMA Oncol 2016;2:508-16. [Crossref] [PubMed]
- American College of Surgeons. About ACS NSQIP. Available online: https://www.facs.org/quality-programs/acs-nsqip/about
- Cohen ME, Hall BL, Liu Y, et al. Evaluating the Benefits of ACS-NSQIP. Ann Surg 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Cohen ME, Ko CY, Bilimoria KY, et al. Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg 2013;217:336-46.e1. [Crossref] [PubMed]
- Fischer JP, Tuggle CT, Au A, et al. A 30-day risk assessment of mastectomy alone compared to immediate breast reconstruction (IBR). J Plast Surg Hand Surg 2014;48:209-15. [Crossref] [PubMed]
- Merkow RP, Kmiecik TE, Bentrem DJ, et al. Effect of including cancer-specific variables on models examining short-term outcomes. Cancer 2013;119:1412-9. [Crossref] [PubMed]
- Merkow RP, Bentrem DJ, Winchester DP, et al. Effect of including cancer-specific variables on risk-adjusted hospital surgical quality comparisons. Ann Surg Oncol 2013;20:1766-73. [Crossref] [PubMed]
- Spolverato G, Ejaz A, Kim Y, et al. Patterns of care among patients undergoing hepatic resection: a query of the National Surgical Quality Improvement Program-targeted hepatectomy database. J Surg Res 2015;196:221-8. [Crossref] [PubMed]


