Bisphosphonate-related osteonecrosis of jaws in advanced stage breast cancer was detected from bone scan: a case report
Introduction
Bisphosphonate-related osteonecrosis of jaw (BRONJ) is defined as an area of exposed bone or bone that can be probed through an intraoral or extraoral fistula in maxillofacial region that has persisted for more than 8 weeks in a patient currently or previously treated with bisphosphonates (BPs) and no history of radiation therapy or obvious metastatic disease to this area (1).
BPs, bone resorption inhibitors, commonly use for treating solid cancer with bone metastasis, such as multiple myeloma, breast cancer and prostate cancer (2). The incidence of BRONJ with intravenous route is of (3.8–9.9%) in multiple myeloma patients, (2.5–2.9%) in breast cancer patients and (2.9–6.5%) in prostate cancer patients (3-5).
In the largest prospective trial collecting 5,723 patients with metastatic bone disease from solid tumors or multiple myeloma receiving either denosumab or zoledronic acid, the incidence of ONJ was 1.6% of the overall patient population and 1.3% of the zoledronic acid group (6).
Managements of those patients were conservative treatment, surgical treatment and adjunct to surgical or non-surgical treatment. Many studies reported various treatment modalities. The purpose of this report is to present a case with BRONJ and deeply review in BRONJ relating topics.
Case presentation
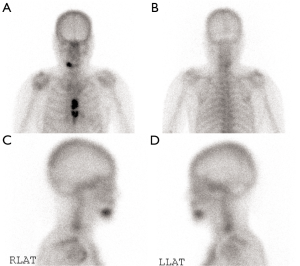
A 79-year-old female Asian patient who presented with left breast mass with ulcer was worked up and diagnosed as advanced stage breast cancer with bony metastasis. Her medical history was chronic kidney disease stage 3, diabetes type 2, hypertension, and dyslipidemia. No history of radiation to head and neck was found. She refused to treat with chemotherapy. Continuing soda mint, phosphate solution and madiplot for her medical problems was done. The specific treatment for advanced stage breast cancer and bone metastasis was arimidex 1 mg/d and zoledronate 3 mg every 4 weeks. Intravenous zoledronate 3 mg q 4 wk (based on patient creatinine clearance) had been administered for three months then creatinine clearance reduced thus we decreased the dosage to 2 mg q 4 wk. We evaluated the bone scan and showed new abnormal increased uptake at the mandible (Figure 1). After later nine treatments, she complained a jaw pain. A complete oral examination was performed and presented as swelling at gingiva (area 43–46) and there was a fistula and pus discharge. Film paronamic and periapical view (Figure 2) showed no remarkable bone destruction and retained dental root 43.

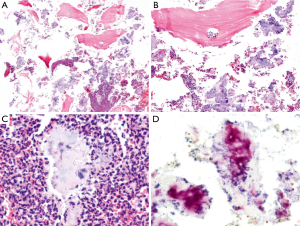
The mandibular treatment was buccal bone decortication, extraction 43, suturing, antibiotics (oral augmentin) and antiseptic mouthwash (0.12% chlorhexidine mouthwash). The final pathology on debridement was shown as fragment of necrotic bone with inflammatory cells infiltration (polymorphonuclear cells predominant) and bacterial colonies with debris tissue (Figure 3A,B,C). The Brown and Brenn stain (Figure 3D) showed gram positive filamentous bacteria. During that the bone was being exposed over 8 weeks. She was followed up to re-evaluate the area until complete healing. The future plan is denture fabrication.
Discussion
Breast cancer with bone metastasis commonly causes osteolytic lesion that depends upon osteoclast-mediated bone resorption (7). BPs are antiresorptive medication to manage hypercalcemia of malignancy and skeletal-related events (SREs) in this condition (4).
BPs are synthetic analogs of pyrophosphate compounds that have the strong affinity for binding to hydroxyapatite (HAP) (8). They can be separated into two general classes according to their chemical structure and molecular mechanism of action. Nitrogen containing BPs that present a nitrogen side chain on pyrophosphate group are commonly prepared for intravenous administration because they are poorly absorbed by gastrointestinal tract about 10%. The nitrogen containing BPs inhibit sterol synthesis via the mevalonate pathway, inducing osteoclast apoptosis (8-10). Non nitrogen containing BPs, which closely relate to pyrophosphate are taken up by osteoclasts and antagonized the cellular energy pathway and integrated into phosphate chain of ATP-containing compounds that accumulate intracellularly to induce apoptosis (8,11). BPs inhibit RANKL expression and enhance osteoprotegerin (OPG) production by bone marrow stromal cells and osteoblasts so that RANK-RANKL interaction is disrupted. These synergistic actions lead to suppression of osteoclast recruitment and bone resorption (11). Currently, there are several proposed theories explaining the pathogenesis of BRONJ.
BPs tend to be highly concentrated in jaws rather than other skeleton sites because of their high vascularity and bone turnover. The forces of masticatory system require a rapid bone turnover and can easily induce microfractures. Additionally, a thin oral mucosa, which separates the jaw from oral environment can be easily traumatized. Oral microbes reach necrotic bone that result in hindering healing process (2,11).
Inhibitory effect of BPs on osteoclasts causes cessation of bone remodeling and bone turnover (11). The potent intravenous BPs used in metastatic patients irreversibly inhibit osteoclasts via interrupting the mevalonate pathway and the result is osteoclast apoptosis (9).
Osteoblasts and osteocytes lifespan is about 150 days. If, upon their death, osteoclasts, which release cytokines of bone morphogenetic protein and insulin-like growth factors to induce new osteoblasts from stem cell population do not absorb mineral matrix, osteon becomes acellular and necrotic (9,12).
BPs could alter blood flow in the mandibular and maxillary bone via inhibition of intraosseous angiogenesis (13). Recent studies have been found that angiogenesis suppression may play a role in developing BRONJ. The strongest decrease in VEGF circulating levels at day 7 and at day 21 after the first administration in BRONJ patients was demonstrated. Therefore, the anti-angiogenic properties of bisphosphonates are directly linked to BRONJ pathogenesis and serum VEGF levels could represent an effective early predictive marker (14). In a recent study by Wehrhan et al., BRONJ and control mucoperiosteal tissue samples were assessed for vascularization with CD31 staining and angiogenesis-related neovessels with CD105 staining. The results showed no significant reduction in CD31-stained capillary area mucoperiosteal BRONJ samples, but significantly fewer CD105-positive vessels in capillary areas than control samples. It indicated that angiogenesis is impaired in BRONJ-related mucoperiosteal tissue, but vascularization remains unaffected. Vessel remodeling and neovessel formation is delayed in BRONJ, resulting in impaired tissue regeneration of bisphosphonate-exposed oral mucosa (15).
Histopathologic features of BRONJ tissue showed three different main histological patterns: non-necrotic areas without inflammation, areas with active acute inflammation and areas with prevalence of necrotic changes. Non-necrotic areas were mostly composed by large masses of bone tissue showing centrifugal deposition and variable degrees of calcification (mature and recent woven bones), devoid of Haversian canals. Islands of woven bone contained plump osteoblasts while osteoclastic activity was absent in such areas. These features suggest that BPs likely act by stimulating appositional osteogenesis without concurrent remodeling or resorption of pre-existing bone trabeculae (16-20). Areas of active acute inflammation contained abundant osteoclast-like cells at the interface with residual bone spiculae and were filled with an inflammatory infiltrate, mostly composed by polymorphonuclear phagocytes, plasma cells, monocytes and lymphocytes, acellular necrotic debris, thin-walled and dilated blood vessels and scattered residual and intensely basophilic bone spiculae. In ONJ affected sites, middle areas were characterized by predominance of bony structure showing wide acellular necrotic regions, large and scalloped Haversian canals and containing inflammatory infiltrates (16).
This case showed ONJ that is an uncommon but serious side effect of Bis administration. The indication was metastatic breast cancer with evidence of bone destruction. She developed ONJ after 12 treatments of IV zoledronate. The diagnosis was made from clinical findings of the lesions that exposed jaw over 8 weeks. Her treatment was dental care, surgery including bone decortication, tooth extraction, sutures, oral antibiotics and antiseptic mouthwash.
Objectives of BRONJ treatment are to eliminate pain, control infection of soft and hard tissue, and minimize progression and occurrence of bone necrosis. The treatment can classify in three categories: conservative treatment, surgical treatment and adjunct to surgical or non-surgical treatment. Conservative treatment is using medication for relieving chronic pain and controlling infection with antibiotics including local such as oral antimicrobial rinses (chlorhexidine 0.12%) and systemic form. Surgical treatment aims to remove bone sequestra by debridement, bone resection and immediate reconstruction with a reconstruction plate or an obturator or vascularized bone. In part of the adjunct to surgical or non-surgical treatment, there are several various options, for example, hyperbaric oxygen therapy (HBO), pentoxifylline and tocopherol, ozone therapy, low level laser therapy (LLLT), and platelet rich plasma (PRP) (1).
HBO might augment burn turnover by producing signal for osteoclast differentiation, activity and viability. A randomized controlled trial of HBO as an adjunct to surgery and antibiotics in BRONJ patients showed that HBO was useful in relieve pain and decreasing lesion size and number at a faster rate than control therapy (standard treatment without HBO, but there was no statistically significant difference in the endpoint of complete gingival coverage). For that reason, authors recommended HBO as adjunctive treatment in severe cases where deep-seated soft tissue infection or refractory osteomyelitis is present (21).
Pentoxifylline and tocopherol have been used in treatment of osteoradionecrosis. Pentoxifylline reduces blood viscosity, improves peripheral blood flow and increase red blood cell membrane flexibility (22). It also has antitumor necrosis factor alpha (TNFα) effect, inhibits dermal fibroblasts and increases collagenase activity (23). Tocopherol is an antioxidant that could influence platelet aggregation, and impairs tissue necrosis. Case series study in using pentoxifylline and tocopherol in addition to antimicrobial therapy showed achieving a 74% decrease in area of bony exposure and symptom control (24).
Ozone enhances tissue oxygenation, improves phagocytosis and diapedesis of phagocytes and stimulates angiogenesis and fibroblasts formation. Furthermore it induces formation of sequestrum, enhances vascularization of the underlying bone and stimulates the formation of granulating tissues (25,26).
LLLT has positive effect in term of improvement and fastening of the wound healing process (27). Two studies have shown higher healing rate in ONJ treatment with LLLT (Nd: YAG) groups than without LLLT groups (27,28). LLLT is a good choice for BRONJ treatment especially for antibacterial and biostimulant properties (29).
PRP is produced by concentrating platelets from whole blood. It contains growth factors that might accelerate epithelial wound healing, decrease tissue inflammation after surgery, improve regeneration of bone and soft tissues, and promote tissue vascularization (30). Several studies indicated positive and successful outcome after adding PRP in BRONJ treatment (31-34).
Currently, there are other novel treatments—autologous bone marrow stem cell, allogenic mesenchymal stem cell therapy. Bone marrow stem cells are known as being multipotent and exhibit the potential for differentiation into different cell/tissue lineages, including cartilage, bone and other tissue. A case report with autologous bone marrow stem cell transplantation into the BRONJ lesion reached complete response (35). Another study in swine, allogenic bone marrow mesenchymal stem cell-based therapy provided a safe and effective therapeutic modality for treating BRONJ condition (36). Lastly, literature review of BRONJ was collected and shown (Table 1).
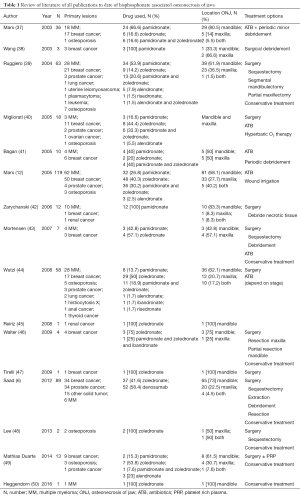
Full table
Conclusions
BPs are used to treat complication of metastatic disease with osteolytic pathology. BRONJ is a rare complication but it is a serious side effect. There is no clinical evidence to support cessation of BPs. In mild cases are mainly treated with conservative treatment. For severe cases may require surgical treatment, such as debridement, resection and reconstruction.
Adjunctive treatment including HBO, pentoxifylline and tocopherol, ozone therapy, LLLT, and PRP are developed and reported in small sample sizes. The efficacy of these treatments needs to be developed in additional research.
Acknowledgements
We wish to acknowledge my clinical fellows team as follows: Dr. Paweena Luadthai, Dr. Suragit Pornchai, Dr. Watoo Vassanasiri, Dr. Noppadol Trikunagonvong and Dr. Chayanoot Rattadilok to encourage these works.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Ruggiero SL, Dodson TB, Fantasia J, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw--2014 update. J Oral Maxillofac Surg 2014;72:1938-56. [Crossref] [PubMed]
- Paulo S, Abrantes AM, Laranjo M, et al. Bisphosphonate-related osteonecrosis of the jaw: specificities. Oncol Rev 2014;8:254. [Crossref] [PubMed]
- Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol 2005;23:8580-7. [Crossref] [PubMed]
- Di Nisio C, Zizzari VL, Zara S, et al. RANK/RANKL/OPG signaling pathways in necrotic jaw bone from bisphosphonate-treated subjects. Eur J Histochem 2015;59:2455. [Crossref] [PubMed]
- Wang EP, Kaban LB, Strewler GJ, et al. Incidence of osteonecrosis of the jaw in patients with multiple myeloma and breast or prostate cancer on intravenous bisphosphonate therapy. J Oral Maxillofac Surg 2007;65:1328-31. [Crossref] [PubMed]
- Saad F, Brown JE, Van Poznak C, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol 2012;23:1341-7. [Crossref] [PubMed]
- Fili S, Karalaki M, Schaller B. Mechanism of bone metastasis: the role of osteoprotegerin and of the host-tissue microenvironment-related survival factors. Cancer Lett 2009;283:10-9. [Crossref] [PubMed]
- Kumar V, Sinha RK. Bisphosphonate Related Osteonecrosis of the Jaw: An Update. J Maxillofac Oral Surg 2014;13:386-93. [Crossref] [PubMed]
- Hewitt C, Farah CS. Bisphosphonate-related osteonecrosis of the jaws: a comprehensive review. J Oral Pathol Med 2007;36:319-28. [Crossref] [PubMed]
- Kumar V, Sinha RK. Evolution and etiopathogenesis of bisphosphonates induced osteonecrosis of the jaw. N Am J Med Sci 2013;5:260-5. [Crossref] [PubMed]
- Sarin J, DeRossi SS, Akintoye SO. Updates on bisphosphonates and potential pathobiology of bisphosphonate-induced jaw osteonecrosis. Oral Dis 2008;14:277-85. [Crossref] [PubMed]
- Marx RE, Sawatari Y, Fortin M, et al. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg 2005;63:1567-75. [Crossref] [PubMed]
- Woo SB, Hellstein JW, Kalmar JR. Narrative (corrected) review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med 2006;144:753-61. [Crossref] [PubMed]
- Vincenzi B, Napolitano A, Zoccoli A, et al. Serum VEGF levels as predictive marker of bisphosphonate-related osteonecrosis of the jaw. J Hematol Oncol 2012;5:56. [Crossref] [PubMed]
- Wehrhan F, Stockmann P, Nkenke E, et al. Differential impairment of vascularization and angiogenesis in bisphosphonate-associated osteonecrosis of the jaw-related mucoperiosteal tissue. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011;112:216-21. [Crossref] [PubMed]
- Favia G, Pilolli GP, Maiorano E. Histologic and histomorphometric features of bisphosphonate-related osteonecrosis of the jaws: an analysis of 31 cases with confocal laser scanning microscopy. Bone 2009;45:406-13. [Crossref] [PubMed]
- Greiner S, Kadow-Romacker A, Schmidmaier G, et al. Cocultures of osteoblasts and osteoclasts are influenced by local application of zoledronic acid incorporated in a poly (D,L-lactide) implant coating. J Biomed Mater Res A 2009;91:288-95. [Crossref] [PubMed]
- Panzavolta S, Torricelli P, Bracci B, et al. Alendronate and Pamidronate calcium phosphate bone cements: setting properties and in vitro response of osteoblast and osteoclast cells. J Inorg Biochem 2009;103:101-6. [Crossref] [PubMed]
- Pampu AA, Dolanmaz D, Tüz HH, et al. Histomorphometric evaluation of the effects of zoledronic acid on mandibular distraction osteogenesis in rabbits. J Oral Maxillofac Surg 2008;66:905-10. [Crossref] [PubMed]
- Boanini E, Torricelli P, Gazzano M, et al. Alendronate-hydroxyapatite nanocomposites and their interaction with osteoclasts and osteoblast-like cells. Biomaterials 2008;29:790-6. [Crossref] [PubMed]
- Freiberger JJ, Padilla-Burgos R, McGraw T, et al. What is the role of hyperbaric oxygen in the management of bisphosphonate-related osteonecrosis of the jaw: a randomized controlled trial of hyperbaric oxygen as an adjunct to surgery and antibiotics. J Oral Maxillofac Surg 2012;70:1573-83. [Crossref] [PubMed]
- Delanian S, Porcher R, Rudant J, et al. Kinetics of response to long-term treatment combining pentoxifylline and tocopherol in patients with superficial radiation-induced fibrosis. J Clin Oncol 2005;23:8570-9. [Crossref] [PubMed]
- Delanian S, Depondt J, Lefaix JL. Major healing of refractory mandible osteoradionecrosis after treatment combining pentoxifylline and tocopherol: a phase II trial. Head Neck 2005;27:114-23. [Crossref] [PubMed]
- Epstein MS, Wicknick FW, Epstein JB, et al. Management of bisphosphonate-associated osteonecrosis: pentoxifylline and tocopherol in addition to antimicrobial therapy. An initial case series. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;110:593-6. [Crossref] [PubMed]
- Agrillo A, Filiaci F, Ramieri V, et al. Bisphosphonate-related osteonecrosis of the jaw (BRONJ): 5 year experience in the treatment of 131 cases with ozone therapy. Eur Rev Med Pharmacol Sci 2012;16:1741-7. [PubMed]
- Bocci V. Ozone as Janus: this controversial gas can be either toxic or medically useful. Mediators Inflamm 2004;13:3-11. [Crossref] [PubMed]
- Vescovi P, Manfredi M, Merigo E, et al. Early surgical laser-assisted management of bisphosphonate-related osteonecrosis of the jaws (BRONJ): a retrospective analysis of 101 treated sites with long-term follow-up. Photomed Laser Surg 2012;30:5-13. [Crossref] [PubMed]
- Vescovi P, Merigo E, Meleti M, et al. Nd:YAG laser biostimulation of bisphosphonate-associated necrosis of the jawbone with and without surgical treatment. Br J Oral Maxillofac Surg 2007;45:628-32. [Crossref] [PubMed]
- Vescovi P, Merigo E, Meleti M, et al. Surgical Approach and Laser Applications in BRONJ Osteoporotic and Cancer Patients. J Osteoporos 2012;2012:585434.
- Albanese A, Licata ME, Polizzi B, et al. Platelet-rich plasma (PRP) in dental and oral surgery: from the wound healing to bone regeneration. Immun Ageing 2013;10:23. [Crossref] [PubMed]
- Cetiner S, Sucak GT, Kahraman SA, et al. Osteonecrosis of the jaw in patients with multiple myeloma treated with zoledronic acid. J Bone Miner Metab 2009;27:435-43. [Crossref] [PubMed]
- Lee CY, David T, Nishime M. Use of platelet-rich plasma in the management of oral biphosphonate-associated osteonecrosis of the jaw: a report of 2 cases. J Oral Implantol 2007;33:371-82. [Crossref] [PubMed]
- Bocanegra-Pérez S, Vicente-Barrero M, Knezevic M, et al. Use of platelet-rich plasma in the treatment of bisphosphonate-related osteonecrosis of the jaw. Int J Oral Maxillofac Surg 2012;41:1410-5. [Crossref] [PubMed]
- Mozzati M, Gallesio G, Arata V, et al. Platelet-rich therapies in the treatment of intravenous bisphosphonate-related osteonecrosis of the jaw: a report of 32 cases. Oral Oncol 2012;48:469-74. [Crossref] [PubMed]
- Cella L, Oppici A, Arbasi M, et al. Autologous bone marrow stem cell intralesional transplantation repairing bisphosphonate related osteonecrosis of the jaw. Head Face Med 2011;7:16. [Crossref] [PubMed]
- Li Y, Xu J, Mao L, et al. Allogeneic mesenchymal stem cell therapy for bisphosphonate-related jaw osteonecrosis in Swine. Stem Cells Dev 2013;22:2047-56. [Crossref] [PubMed]
- Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg 2003;61:1115-7. [Crossref] [PubMed]
- Wang J, Goodger NM, Pogrel MA. Osteonecrosis of the jaws associated with cancer chemotherapy. J Oral Maxillofac Surg 2003;61:1104-7. [Crossref] [PubMed]
- Ruggiero SL, Mehrotra B, Rosenberg TJ, et al. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg 2004;62:527-34. [Crossref] [PubMed]
- Migliorati CA, Schubert MM, Peterson DE, et al. Bisphosphonate-associated osteonecrosis of mandibular and maxillary bone: an emerging oral complication of supportive cancer therapy. Cancer 2005;104:83-93. [Crossref] [PubMed]
- Bagan JV, Murillo J, Jimenez Y, et al. Avascular jaw osteonecrosis in association with cancer chemotherapy: series of 10 cases. J Oral Pathol Med 2005;34:120-3. [Crossref] [PubMed]
- Zarychanski R, Elphee E, Walton P, et al. Osteonecrosis of the jaw associated with pamidronate therapy. Am J Hematol 2006;81:73-5. [Crossref] [PubMed]
- Mortensen M, Lawson W, Montazem A. Osteonecrosis of the jaw associated with bisphosphonate use: Presentation of seven cases and literature review. Laryngoscope 2007;117:30-4. [Crossref] [PubMed]
- Wutzl A, Biedermann E, Wanschitz F, et al. Treatment results of bisphosphonate-related osteonecrosis of the jaws. Head Neck 2008;30:1224-30. [Crossref] [PubMed]
- Reiriz AB, De Zorzi Pde M, Lovat CP. Bisphosphonates and osteonecrosis of the jaw: a case report. Clinics (Sao Paulo) 2008;63:281-4. [Crossref] [PubMed]
- Walter C, Al-Nawas B, du Bois A, et al. Incidence of bisphosphonate-associated osteonecrosis of the jaws in breast cancer patients. Cancer 2009;115:1631-7. [Crossref] [PubMed]
- Tirelli G, Biasotto M, Chiandussi S, et al. Bisphosphonate-associated osteonecrosis of the jaws: the limits of a conservative approach. Head Neck 2009;31:1249-54. [Crossref] [PubMed]
- Lee JJ, Cheng SJ, Wang YP, et al. Osteonecrosis of the jaws associated with the use of yearly zoledronic acid: report of 2 cases. Head Neck 2013;35:E6-10. [Crossref] [PubMed]
- Mathias Duarte LF, dos Reis HB, Tucci R, et al. Bisphosphonate-related osteonecrosis of the jaws: analysis of a case series at a dental school. Spec Care Dentist 2014;34:77-83. [Crossref] [PubMed]
- Heggendorn FL, Leite TC, Cunha KS, et al. Bisphosphonate-related osteonecrosis of the jaws: Report of a case using conservative protocol. Spec Care Dentist 2016;36:43-7. [Crossref] [PubMed]


